A Phase III Study of IncobotulinumtoxinA in the Treatment of Glabellar Frown Lines
- PMID: 22010053
- PMCID: PMC3196303
A Phase III Study of IncobotulinumtoxinA in the Treatment of Glabellar Frown Lines
Abstract
Objective: To investigate the efficacy and safety of incobotulinumtoxinA (also known as botulinum toxin type A [150 kDa], free from complexing proteins, or previously as NT 201), for the treatment of glabellar frown lines, in a prospective, open-label, multicenter, Phase III trial.
Design: The study was a prospective, open-label, multicenter, international, Phase III clinical study. Subjects with moderate-to-severe glabellar frown lines at maximum frown, as assessed by the investigator according to the facial wrinkle scale, were given one intramuscular treatment of 20U incobotulinumtoxinA, administered as 0. 1mL to each of five injection sites, and assessed over 84 days. Missing values were imputed using the baseline value or next observation carried backwards. Adverse events were documented for the duration of the study.
Settings: Two centers in Russia and one center in Germany.
Participants: A total of 105 subjects (18-65 years) with moderate-to-severe glabellar frown lines at maximum frown were enrolled.
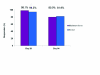
Measurement: The primary endpoint was the percentage of responders at maximum frown (improvement of ≥1 on the facial wrinkle scale when compared with Day 0) on Day 28, as assessed by the investigator.
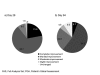
Results: Response to treatment with incobotulinumtoxinA with respect to the facial wrinkle scale at maximum frown on Day 28 and Day 84 was 98.1 and 80.0 percent, respectively (missing values imputed). At rest, 94.3 percent (imputed values) of subjects were responders on Day 28 while 81.9 percent were responders on Day 84 (imputed values). Consistent with assessment by the investigators, subjects also rated treatment success highly. Incidence of treatment-related adverse events was low and no patients developed neutralizing antibodies.
Conclusion: IncobotulinumtoxinA is effective in the treatment of glabellar frown lines and is well tolerated.
Figures


References
-
- Carruthers A, Carruthers J. Botulinum toxin products overview. Skin Therapy Lett. 2008;13:1–4. - PubMed
-
- Carruthers JD, Carruthers JA. Treatment of glabellar frown lines with C. botulinum-A exotoxin. J Dermatol Surg Oncol. 1992;18:17–21. - PubMed
-
- Ascher B, Zakine B, Kestemont P, et al. A multicenter, randomized, double-blind, placebo-controlled study of efficacy and safety of 3 doses of botulinum toxin A in the treatment of glabellar lines. JAmAcad Dermatol. 2004;51:223–233. - PubMed
-
- Carruthers A, Carruthers J, Lowe NJ, et al. One-year, randomised, multicenter, two-period study of the safety and efficacy of repeated treatments with botulinum toxin type A in patients with glabellar lines. J Clin Res. 2004;7:1–20.
-
- Carruthers A, Carruthers J, Said S. Dose-ranging study of botulinum toxin type A in the treatment of glabellar rhytids in females. Dermatol Surg. 2005;31:414–422. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
