Dominant and pleiotropic effects of a GAI gene in wheat results from a lack of interaction between DELLA and GID1
- PMID: 22010107
- PMCID: PMC3327208
- DOI: 10.1104/pp.111.185272
Dominant and pleiotropic effects of a GAI gene in wheat results from a lack of interaction between DELLA and GID1
Abstract
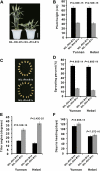
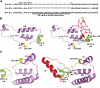
Dominance, semidominance, and recessiveness are important modes of Mendelian inheritance. The phytohormone gibberellin (GA) regulates many plant growth and developmental processes. The previously cloned semidominant GA-insensitive (GAI) genes Reduced height1 (Rht1) and Rht2 in wheat (Triticum aestivum) were the basis of the Green Revolution. However, no completely dominant GAI gene has been cloned. Here, we report the molecular characterization of Rht-B1c, a dominant GAI allele in wheat that confers more extreme characteristics than its incompletely dominant alleles. Rht-B1c is caused by a terminal repeat retrotransposons in miniature insertion in the DELLA domain. Yeast two-hybrid assays showed that Rht-B1c protein fails to interact with GA-INSENSITIVE DWARF1 (GID1), thereby blocking GA responses and resulting in extreme dwarfism and pleiotropic effects. By contrast, Rht-B1b protein only reduces interaction with GID1. Furthermore, we analyzed its functions using near-isogenic lines and examined its molecular mechanisms in transgenic rice. These results indicated that the affinity between GID1 and DELLA proteins is key to regulation of the stability of DELLA proteins, and differential interactions determine dominant and semidominant gene responses to GA.
Figures
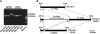



References
-
- Achard P, Herr A, Baulcombe DC, Harberd NP. (2004) Modulation of floral development by a gibberellin-regulated microRNA. Development 131: 3357–3365 - PubMed
-
- Antonius-Klemola K, Kalendar R, Schulman AH. (2006) TRIM retrotransposons occur in apple and are polymorphic between varieties but not sports. Theor Appl Genet 112: 999–1008 - PubMed
-
- Appleford NEJ, Wilkinson MD, Ma Q, Evans DJ, Stone MC, Pearce SP, Powers SJ, Thomas SG, Jones HD, Phillips AL, et al. (2007) Decreased shoot stature and grain α-amylase activity following ectopic expression of a gibberellin 2-oxidase gene in transgenic wheat. J Exp Bot 58: 3213–3226 - PubMed
-
- Borlaug NE. (1983) Contributions of conventional plant breeding to food production. Science 219: 689–693 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

