Thyroid dysfunction from antineoplastic agents
- PMID: 22010182
- PMCID: PMC3206040
- DOI: 10.1093/jnci/djr373
Thyroid dysfunction from antineoplastic agents
Abstract
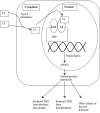
Unlike cytotoxic agents that indiscriminately affect rapidly dividing cells, newer antineoplastic agents such as targeted therapies and immunotherapies are associated with thyroid dysfunction. These include tyrosine kinase inhibitors, bexarotene, radioiodine-based cancer therapies, denileukin diftitox, alemtuzumab, interferon-α, interleukin-2, ipilimumab, tremelimumab, thalidomide, and lenalidomide. Primary hypothyroidism is the most common side effect, although thyrotoxicosis and effects on thyroid-stimulating hormone secretion and thyroid hormone metabolism have also been described. Most agents cause thyroid dysfunction in 20%-50% of patients, although some have even higher rates. Despite this, physicians may overlook drug-induced thyroid dysfunction because of the complexity of the clinical picture in the cancer patient. Symptoms of hypothyroidism, such as fatigue, weakness, depression, memory loss, cold intolerance, and cardiovascular effects, may be incorrectly attributed to the primary disease or to the antineoplastic agent. Underdiagnosis of thyroid dysfunction can have important consequences for cancer patient management. At a minimum, the symptoms will adversely affect the patient's quality of life. Alternatively, such symptoms can lead to dose reductions of potentially life-saving therapies. Hypothyroidism can also alter the kinetics and clearance of medications, which may lead to undesirable side effects. Thyrotoxicosis can be mistaken for sepsis or a nonendocrinologic drug side effect. In some patients, thyroid disease may indicate a higher likelihood of tumor response to the agent. Both hypothyroidism and thyrotoxicosis are easily diagnosed with inexpensive and specific tests. In many patients, particularly those with hypothyroidism, the treatment is straightforward. We therefore recommend routine testing for thyroid abnormalities in patients receiving these antineoplastic agents.
Figures



Comment in
-
Re: Thyroid dysfunction from antineoplastic agents.J Natl Cancer Inst. 2012 Mar 7;104(5):422-3; author reply 423. doi: 10.1093/jnci/djs011. Epub 2012 Jan 30. J Natl Cancer Inst. 2012. PMID: 22291212 No abstract available.
References
-
- van der Molen AJ, Thomsen HS, Morcos SK. Effect of iodinated contrast media on thyroid function in adults. Eur Radiol. 2004;14(5):902–907. - PubMed
-
- Markou K, Georgopoulos N, Kyriazopoulou V, Vagenakis AG. Iodine-induced hypothyroidism. Thyroid. 2001;11(5):501–510. - PubMed
-
- Berger C, Le-Gallo B, Donadieu J, et al. Late thyroid toxicity in 153 long-term survivors of allogeneic bone marrow transplantation for acute lymphoblastic leukaemia. Bone Marrow Transplant. 2005;35(10):991–995. - PubMed
-
- Jereczek-Fossa BA, Alterio D, Jassem J, et al. Radiotherapy-induced thyroid disorders. Cancer Treat Rev. 2004;30(4):369–384. - PubMed
-
- Schneider AB, Sarne DH. Long-term risks for thyroid cancer and other neoplasms after exposure to radiation. Nat Clin Pract Endocrinol Metab. 2005;1(2):82–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

