Biochemical and thermodynamic analyses of Salmonella enterica Pat, a multidomain, multimeric N(ε)-lysine acetyltransferase involved in carbon and energy metabolism
- PMID: 22010215
- PMCID: PMC3195501
- DOI: 10.1128/mBio.00216-11
Biochemical and thermodynamic analyses of Salmonella enterica Pat, a multidomain, multimeric N(ε)-lysine acetyltransferase involved in carbon and energy metabolism
Abstract
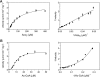
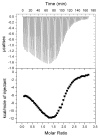
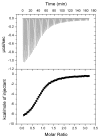
In the bacterium Salmonella enterica, the CobB sirtuin protein deacetylase and the Gcn5-related N(ε)-acetyltransferase (GNAT) Pat control carbon utilization and metabolic flux via N(ε)-lysine acetylation/deacetylation of metabolic enzymes. To date, the S. enterica Pat (SePat) acetyltransferase has not been biochemically characterized. Here we report the kinetic and thermodynamic characterization of the SePat enzyme using two of its substrates, acetyl coenzyme A (Ac-CoA) synthetase (Acs; AMP forming, EC 6.2.1.1) and Ac-CoA. The data showed typical Michaelis-Menten kinetic behavior when Ac-CoA was held at a saturating concentration while Acs was varied, and a sigmoidal kinetic behavior was observed when Acs was saturating and the Ac-CoA concentration was varied. The observation of sigmoidal kinetics and positive cooperativity for Ac-CoA is an unusual feature of GNATs. Results of isothermal titration calorimetry (ITC) experiments showed that binding of Ac-CoA to wild-type SePat produced a biphasic curve having thermodynamic properties consistent with two distinct sites. Biphasicity was not observed in ITC experiments that analyzed the binding of Ac-CoA to a C-terminal construct of SePat encompassing the predicted core acetyltransferase domain. Subsequent analytical gel filtration chromatography studies showed that in the presence of Ac-CoA, SePat oligomerized to a tetrameric form, whereas in the absence of Ac-CoA, SePat behaved as a monomer. The positive modulation of SePat activity by Ac-CoA, a product of the Acs enzyme that also serves as a substrate for SePat-dependent acetylation, is likely a layer of metabolic control. IMPORTANCE For decades, N(ε)-lysine acetylation has been a well-studied mode of regulation of diverse proteins involved in almost all aspects of eukaryotic physiology. Until recently, N(ε)-lysine acetylation was not considered a widespread phenomenon in bacteria. Recent studies have indicated that N(ε)-lysine acetylation and its impact on cellular metabolism may be just as diverse in bacteria as they are in eukaryotes. The S. enterica Pat enzyme, specifically, has recently been implicated in the modulation of many metabolic enzymes. Understanding the molecular mechanisms of how this enzyme controls the activity of diverse enzymes by N(ε)-lysine acetylation will advance our understanding of how the prokaryotic cell responds to its changing environment in order to meet its metabolic needs.
Figures


References
-
- Neuwald AF, Landsman D. 1997. GCN5-related histone N-acetyltransferases belong to a diverse superfamily that includes the yeast SPT10 protein. Trends Biochem. Sci. 22:154–155 - PubMed
-
- Vetting MW, et al. 2005. Structure and functions of the GNAT superfamily of acetyltransferases. Arch. Biochem. Biophys. 433:212–226 - PubMed
-
- Allis CD, et al. 2007. New nomenclature for chromatin-modifying enzymes. Cell 131:633–636 - PubMed
-
- Kouzarides T. 1999. Histone acetylases and deacetylases in cell proliferation. Curr. Opin. Genet. Dev. 9:40–48 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
