Prediction of relative in vivo metabolite exposure from in vitro data using two model drugs: dextromethorphan and omeprazole
- PMID: 22010218
- PMCID: PMC3250047
- DOI: 10.1124/dmd.111.042200
Prediction of relative in vivo metabolite exposure from in vitro data using two model drugs: dextromethorphan and omeprazole
Abstract
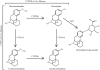
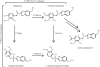
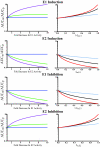
Metabolites can have pharmacological or toxicological effects, inhibit metabolic enzymes, and be used as probes of drug-drug interactions or specific cytochrome P450 (P450) phenotypes. Thus, better understanding and prediction methods are needed to characterize metabolite exposures in vivo. This study aimed to test whether in vitro data could be used to predict and rationalize in vivo metabolite exposures using two model drugs and P450 probes: dextromethorphan and omeprazole with their primary metabolites dextrorphan, 5-hydroxyomeprazole (5OH-omeprazole), and omeprazole sulfone. Relative metabolite exposures were predicted using metabolite formation and elimination clearances. For dextrorphan, the formation clearances of dextrorphan glucuronide and 3-hydroxymorphinan from dextrorphan in human liver microsomes were used to predict metabolite (dextrorphan) clearance. For 5OH-omeprazole and omeprazole sulfone, the depletion rates of the metabolites in human hepatocytes were used to predict metabolite clearance. Dextrorphan/dextromethorphan in vivo metabolite/parent area under the plasma concentration versus time curve ratio (AUC(m)/AUC(p)) was overpredicted by 2.1-fold, whereas 5OH-omeprazole/omeprazole and omeprazole sulfone/omeprazole were predicted within 0.75- and 1.1-fold, respectively. The effect of inhibition or induction of the metabolite's formation and elimination on the AUC(m)/AUC(p) ratio was simulated. The simulations showed that unless metabolite clearance pathways are characterized, interpretation of the metabolic ratios is exceedingly difficult. This study shows that relative in vivo metabolite exposure can be predicted from in vitro data and characterization of secondary metabolism of probe metabolites is critical for interpretation of phenotypic data.
Figures



Similar articles
-
Multiple human cytochromes contribute to biotransformation of dextromethorphan in-vitro: role of CYP2C9, CYP2C19, CYP2D6, and CYP3A.J Pharm Pharmacol. 1998 Sep;50(9):997-1004. doi: 10.1111/j.2042-7158.1998.tb06914.x. J Pharm Pharmacol. 1998. PMID: 9811160
-
Metabolism of dextromethorphan in human liver microsomes: a rapid HPCL assay to monitor cytochrome P450 2D6 activity.Pharmazie. 1996 Aug;51(8):586-8. Pharmazie. 1996. PMID: 8794469
-
Comparative contribution to dextromethorphan metabolism by cytochrome P450 isoforms in vitro: can dextromethorphan be used as a dual probe for both CTP2D6 and CYP3A activities?Drug Metab Dispos. 2001 Nov;29(11):1514-20. Drug Metab Dispos. 2001. PMID: 11602530
-
Pharmacokinetic studies with esomeprazole, the (S)-isomer of omeprazole.Clin Pharmacokinet. 2001;40(6):411-26. doi: 10.2165/00003088-200140060-00003. Clin Pharmacokinet. 2001. PMID: 11475467 Review.
-
Rationalization and prediction of in vivo metabolite exposures: the role of metabolite kinetics, clearance predictions and in vitro parameters.Expert Opin Drug Metab Toxicol. 2010 Sep;6(9):1095-109. doi: 10.1517/17425255.2010.497487. Expert Opin Drug Metab Toxicol. 2010. PMID: 20557268 Free PMC article. Review.
Cited by
-
Effect of isotretinoin on CYP2D6 and CYP3A activity in patients with severe acne.Br J Clin Pharmacol. 2024 Mar;90(3):759-768. doi: 10.1111/bcp.15938. Epub 2023 Nov 21. Br J Clin Pharmacol. 2024. PMID: 37864393 Free PMC article.
-
Hepatic Cyp2d and Cyp26a1 mRNAs and activities are increased during mouse pregnancy.Drug Metab Dispos. 2013 Feb;41(2):312-9. doi: 10.1124/dmd.112.049379. Epub 2012 Nov 13. Drug Metab Dispos. 2013. PMID: 23150428 Free PMC article.
-
In vitro analysis and quantitative prediction of efavirenz inhibition of eight cytochrome P450 (CYP) enzymes: major effects on CYPs 2B6, 2C8, 2C9 and 2C19.Drug Metab Pharmacokinet. 2013;28(4):362-71. doi: 10.2133/dmpk.dmpk-12-rg-124. Epub 2013 Feb 5. Drug Metab Pharmacokinet. 2013. PMID: 23385314 Free PMC article.
-
Effects of elagolix on the pharmacokinetics of omeprazole and its metabolites in healthy premenopausal women.Clin Transl Sci. 2022 May;15(5):1269-1280. doi: 10.1111/cts.13247. Epub 2022 Mar 2. Clin Transl Sci. 2022. PMID: 35137535 Free PMC article.
-
Physiologically based pharmacokinetic (PBPK) modeling of the role of CYP2D6 polymorphism for metabolic phenotyping with dextromethorphan.Front Pharmacol. 2022 Oct 24;13:1029073. doi: 10.3389/fphar.2022.1029073. eCollection 2022. Front Pharmacol. 2022. PMID: 36353484 Free PMC article.
References
-
- Abduljalil K, Frank D, Gaedigk A, Klaassen T, Tomalik-Scharte D, Jetter A, Jaehde U, Kirchheiner J, Fuhr U. (2010) Assessment of activity levels for CYP2D6*1, CYP2D6*2, and CYP2D6*41 genes by population pharmacokinetics of dextromethorphan. Clin Pharmacol Ther 88:643–651 - PubMed
-
- Anderson S, Luffer-Atlas D, Knadler MP. (2009) Predicting circulating human metabolites: how good are we? Chem Res Toxicol 22:243–256 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

