Metal ions: supporting actors in the playbook of small ribozymes
- PMID: 22010272
- PMCID: PMC3365584
- DOI: 10.1039/9781849732512-00175
Metal ions: supporting actors in the playbook of small ribozymes
Abstract
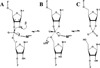
Since the 1980s, several small RNA motifs capable of chemical catalysis have been discovered. These small ribozymes, composed of between approximately 40 and 200 nucleotides, have been found to play vital roles in the replication of subviral and viral pathogens, as well as in gene regulation in prokaryotes, and have recently been discovered in noncoding eukaryotic RNAs. All of the known natural small ribozymes - the hairpin, hammerhead, hepatitis delta virus, Varkud satellite, and glmS ribozymes--catalyze the same self-cleavage reaction as RNase A, resulting in two products, one bearing a 2'-3' cyclic phosphate and the other a 5'-hydroxyl group. Although originally thought to be obligate metalloenzymes like the group I and II self-splicing introns, the small ribozymes are now known to support catalysis in a wide variety of cations that appear to be only indirectly involved in catalysis. Nevertheless, under physiologic conditions, metal ions are essential for the proper folding and function of the small ribozymes, the most effective of these being magnesium. Metal ions contribute to catalysis in the small ribozymes primarily by stabilizing the catalytically active conformation, but in some cases also by activating RNA functional groups for catalysis, directly participating in catalytic acid-base chemistry, and perhaps by neutralizing the developing negative charge of the transition state. Although interactions between the small ribozymes and cations are relatively nonspecific, ribozyme activity is quite sensitive to the types and concentrations of metal ions present in solution, suggesting a close evolutionary relationship between cellular metal ion homeostasis and cation requirements of catalytic RNAs, and perhaps RNA in general.
Figures
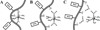
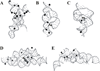
References
-
- Kruger K, Grabowski PJ, Zaug AJ, Sands J, Gottschling DE, Cech TR. Cell. 1982;31:147–157. - PubMed
-
- Cech TR. Science. 2000;289:878–879. - PubMed
-
- Guerrier-Takada C, Gardiner K, Marsh T, Pace N, Altman S. Cell. 1983;35:849–857. - PubMed
-
- Winkler WC, Nahvi A, Roth A, Collins JA, Breaker RR. Nature. 2004;428:281–286. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
