Metal-free intermolecular oxidative C-N bond formation via tandem C-H and N-H bond functionalization
- PMID: 22010982
- PMCID: PMC3233640
- DOI: 10.1021/ja2087085
Metal-free intermolecular oxidative C-N bond formation via tandem C-H and N-H bond functionalization
Abstract
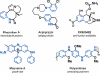
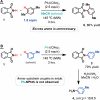
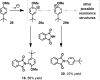
The development of a novel intermolecular oxidative amination reaction, a synthetic transformation that involves the simultaneous functionalization of both a N-H and C-H bond, is described. The process, which is mediated by an I(III) oxidant and contains no metal catalysts, provides a rapid and green method for synthesizing protected anilines from simple arenes and phthalimide. Mechanistic investigations indicate that the reaction proceeds via nucleophilic attack of the phthalimide on an aromatic radical cation, as opposed to the electrophilic aromatic amination that has been reported for other I(III) amination reactions. The application of this new reaction to the synthesis of a variety of substituted aniline derivatives is demonstrated.
© 2011 American Chemical Society
Figures



References
-
-
For recent reviews describing oxidative C-C formation, see: Cho SH, Kim JY, Kwak J, Chang S. Chem. Soc. Rev. 2011;40:5068–5083.Yeung CS, Dong VM. Chem. Rev. 2011;111:1215.McGlacken GP, Bateman LM. Chem. Soc. Rev. 2009;38:2447.
-
-
-
For recent reviews describing C-N formation via nitreneoid intermediates, see: Davies HML, Manning JR. Nature. 2008;451:417.Müller P, Fruit C. Chem. Rev. 2003;103:2905.
-
-
-
For a partial review, see: ref. , pp. 12-16.
-
-
-
For recent examples of Cu and Pd-catalyzed aminations, see: Tsang WCP, Zheng N, Buchwald SL. J. Am. Chem. Soc. 2005;127:14560.Thu H-Y, Yu W-Y, Che CM. J. Am. Chem. Soc. 2006;128:9048.Tsang WCP, Munday RH, Brasche G, Zheng N, Buchwald SL. J. Org. Chem. 2008;73:7603.Li B, Tian S, Fang Z, Shi Z. Angew. Chem. Int. Ed. 2008;47:1115.Jordan-Hore JA, Johansson CCC, Beck EM, Gaunt MJ. J. Am. Chem. Soc. 2008;130:16184.Miura T, Murakami M. Chem. Lett. 2009;38:328.John A, Nicholas KM. J. Org. Chem. 2011;76:4158.Xiao B, Gong T-J, Xu J, Liu Z-J, Liu L. J. Am. Chem. Soc. 2011;133:1466.Sun K, Li Y, Xiong T, Zhang J, Zhan Q. J. Am. Chem. Soc. 2011;133:1694.Yoo EJ, Ma S, Mei T-S, Chan KSL, Yu J-Q. J. Am. Chem. Soc. 2011;133:7652.
-
-
-
For a recent examples of a metal-free amination, see: Cho SH, Yoon J, Chang S. J. Am. Chem. Soc. 2011;133:5696.Lamani M, Prabhu KR. Iodine-Catalyzed Amination of Benzoxazoles: A Metal-Free Route to 2-Aminobenzoxazoles under Mild Conditions. J. Org. Chem. 2011 [Online early access]. DOI: 10.1021/jo201402a. PublishedOnline: Aug 25, 2011.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

