Overcoming endosomal barrier by amphotericin B-loaded dual pH-responsive PDMA-b-PDPA micelleplexes for siRNA delivery
- PMID: 22011045
- PMCID: PMC4797624
- DOI: 10.1021/nn203503h
Overcoming endosomal barrier by amphotericin B-loaded dual pH-responsive PDMA-b-PDPA micelleplexes for siRNA delivery
Abstract
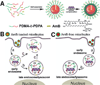
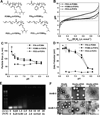
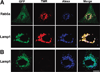
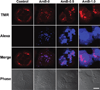
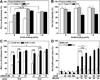
The endosomal barrier is a major bottleneck for the effective intracellular delivery of siRNA by nonviral nanocarriers. Here, we report a novel amphotericin B (AmB)-loaded, dual pH-responsive micelleplex platform for siRNA delivery. Micelles were self-assembled from poly(2-(dimethylamino)ethyl methacrylate)-block-poly(2-(diisopropylamino)ethyl methacrylate) (PDMA-b-PDPA) diblock copolymers. At pH 7.4, AmB was loaded into the hydrophobic PDPA core, and siRNA was complexed with a positively charged PDMA shell to form the micelleplexes. After cellular uptake, the PDMA-b-PDPA/siRNA micelleplexes dissociated in early endosomes to release AmB. Live cell imaging studies demonstrated that released AmB significantly increased the ability of siRNA to overcome the endosomal barrier. Transfection studies showed that AmB-loaded micelleplexes resulted in significant increase in luciferase (Luc) knockdown efficiency over the AmB-free control. The enhanced Luc knockdown efficiency was abolished by bafilomycin A1, a vacuolar ATPase inhibitor that inhibits the acidification of the endocytic organelles. These data support the central hypothesis that membrane poration by AmB and increased endosomal swelling and membrane tension by a "proton sponge" polymer provided a synergistic strategy to disrupt endosomes for improved intracellular delivery of siRNA.
© 2011 American Chemical Society
Figures
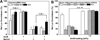
References
-
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-Nucleotide RNAs Mediate RNA Interference in Cultured Mammalian Cells. Nature. 2001;411:494–498. - PubMed
-
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and Specific Genetic Interference by Double-Stranded RNA in Caenorhabditis Elegans. Nature. 1998;391:806–811. - PubMed
-
- Hannon GJ, Rossi JJ. Unlocking the Potential of the Human Genome with RNA Interference. Nature. 2004;431:371–378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

