Chitosan enhances mineralization during osteoblast differentiation of human bone marrow-derived mesenchymal stem cells, by upregulating the associated genes
- PMID: 22011046
- PMCID: PMC6496487
- DOI: 10.1111/j.1365-2184.2011.00788.x
Chitosan enhances mineralization during osteoblast differentiation of human bone marrow-derived mesenchymal stem cells, by upregulating the associated genes
Abstract
Objectives: Chitosan is widely used as a scaffold for bone tissue engineering. However, up-to-date, no previous detailed study has been conducted to elucidate any mechanism of osteogenesis by chitosan itself. Here, we have evaluated effects of chitosan-coated tissue culture plates on adhesion and osteoblast differentiation processes of human mesenchymal stem cells (hMSCs), isolated from adult bone marrow.
Materials and methods: Tissue culture plates coated with chitosan at different coating densities were used to evaluate the effects on hMSC adhesion and osteoblast differentiation. hMSCs were induced to differentiate into osteoblasts on the chitosan-coated plates and were evaluated using established techniques: alkaline phosphatase assay, demonstration of presence of calcium and real time PCR.
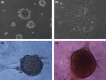
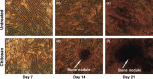
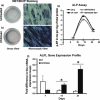
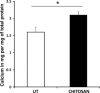
Results: The cells adhered to plates of lower coating density of chitosan, but formed viable cell aggregates at higher coating density (100 μg/sq.cm). Coating density of 25 μg/sq.cm, supporting cell adhesion was chosen for osteoblast differentiation experiments. Differentiating hMSCs showed higher mineral deposition and calcium content on chitosan-coated plates. Chitosan upregulated genes associated with calcium binding and mineralization such as collagen type 1 alpha 1, integrin-binding sialoprotein, osteopontin, osteonectin and osteocalcin, significantly.
Conclusions: We demonstrate for the first time that chitosan enhanced mineralization by upregulating the associated genes. Thus, the study may help clinical situations promoting use of chitosan in bone mineralization, necessary for healing non-union fractures and more.
© 2011 Blackwell Publishing Ltd.
Figures



Similar articles
-
A novel tripolymer coating demonstrating the synergistic effect of chitosan, collagen type 1 and hyaluronic acid on osteogenic differentiation of human bone marrow derived mesenchymal stem cells.Biochem Biophys Res Commun. 2011 Oct 14;414(1):270-6. doi: 10.1016/j.bbrc.2011.09.071. Epub 2011 Sep 17. Biochem Biophys Res Commun. 2011. PMID: 21951845
-
Growth of mesenchymal stem cells on electrospun type I collagen nanofibers.Stem Cells. 2006 Nov;24(11):2391-7. doi: 10.1634/stemcells.2006-0253. Stem Cells. 2006. PMID: 17071856
-
Extracellular matrix protein mediated regulation of the osteoblast differentiation of bone marrow derived human mesenchymal stem cells.Differentiation. 2012 Sep;84(2):185-92. doi: 10.1016/j.diff.2012.05.001. Epub 2012 Jun 2. Differentiation. 2012. PMID: 22664173
-
Controlled Dual Growth Factor Delivery From Microparticles Incorporated Within Human Bone Marrow-Derived Mesenchymal Stem Cell Aggregates for Enhanced Bone Tissue Engineering via Endochondral Ossification.Stem Cells Transl Med. 2016 Feb;5(2):206-17. doi: 10.5966/sctm.2015-0115. Epub 2015 Dec 23. Stem Cells Transl Med. 2016. PMID: 26702127 Free PMC article.
-
The effect of five proteins on stem cells used for osteoblast differentiation and proliferation: a current review of the literature.Cell Mol Life Sci. 2014 Jan;71(1):113-42. doi: 10.1007/s00018-013-1326-0. Epub 2013 Apr 9. Cell Mol Life Sci. 2014. PMID: 23568025 Free PMC article. Review.
Cited by
-
Microcarrier culture for efficient expansion and osteogenic differentiation of human fetal mesenchymal stem cells.Biores Open Access. 2013 Apr;2(2):84-97. doi: 10.1089/biores.2013.0001. Biores Open Access. 2013. PMID: 23593561 Free PMC article.
-
Gold nanoparticles promote osteogenic differentiation in human adipose-derived mesenchymal stem cells through the Wnt/β-catenin signaling pathway.Int J Nanomedicine. 2015 Jul 7;10:4383-92. doi: 10.2147/IJN.S78775. eCollection 2015. Int J Nanomedicine. 2015. PMID: 26185441 Free PMC article.
-
Delivery of bioactive lipids from composite microgel-microsphere injectable scaffolds enhances stem cell recruitment and skeletal repair.PLoS One. 2014 Jul 31;9(7):e101276. doi: 10.1371/journal.pone.0101276. eCollection 2014. PLoS One. 2014. PMID: 25077607 Free PMC article.
-
Optimizing Delivery of Therapeutic Growth Factors for Bone and Cartilage Regeneration.Gels. 2023 May 3;9(5):377. doi: 10.3390/gels9050377. Gels. 2023. PMID: 37232969 Free PMC article. Review.
-
Injectable nanoporous microgels generate vascularized constructs and support bone regeneration in critical-sized defects.Sci Rep. 2022 Sep 22;12(1):15811. doi: 10.1038/s41598-022-19968-x. Sci Rep. 2022. PMID: 36138042 Free PMC article.
References
-
- Johnell O, Kanis JA (2006) An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 17, 1726–1733. - PubMed
-
- Salgado AJ, Coutinho OP, Reis RL (2004) Bone tissue engineering: state of the art and future trends. Macromol. Biosci. 4, 743–765. - PubMed
-
- Bajada S, Mazakova I, Richardson JB, Ashammakhi N (2008) Updates on stem cells and their applications in regenerative medicine. J. Tissue Eng. Regen. Med. 2, 169–183. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

