Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers
- PMID: 22011213
- PMCID: PMC3313609
- DOI: 10.1089/ten.TEA.2011.0391
Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers
Abstract
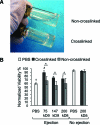
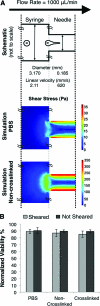
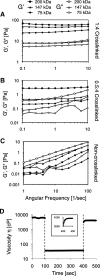
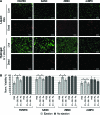
Cell transplantation is a promising therapy for a myriad of debilitating diseases; however, current delivery protocols using direct injection result in poor cell viability. We demonstrate that during the actual cell injection process, mechanical membrane disruption results in significant acute loss of viability at clinically relevant injection rates. As a strategy to protect cells from these damaging forces, we hypothesize that cell encapsulation within hydrogels of specific mechanical properties will significantly improve viability. We use a controlled in vitro model of cell injection to demonstrate success of this acute protection strategy for a wide range of cell types including human umbilical vein endothelial cells (HUVEC), human adipose stem cells, rat mesenchymal stem cells, and mouse neural progenitor cells. Specifically, alginate hydrogels with plateau storage moduli (G') ranging from 0.33 to 58.1 Pa were studied. A compliant crosslinked alginate hydrogel (G'=29.6 Pa) yielded the highest HUVEC viability, 88.9% ± 5.0%, while Newtonian solutions (i.e., buffer only) resulted in 58.7% ± 8.1% viability. Either increasing or decreasing the hydrogel storage modulus reduced this protective effect. Further, cells within noncrosslinked alginate solutions had viabilities lower than media alone, demonstrating that the protective effects are specifically a result of mechanical gelation and not the biochemistry of alginate. Experimental and theoretical data suggest that extensional flow at the entrance of the syringe needle is the main cause of acute cell death. These results provide mechanistic insight into the role of mechanical forces during cell delivery and support the use of protective hydrogels in future clinical stem cell injection studies.
Figures
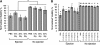
References
-
- Laflamme M.A. Chen K.Y. Naumova A.V. Muskheli V. Fugate J.A. Dupras S.K. Reinecke H. Xu C. Hassanipour M. Police S. O'Sullivan C. Collins L. Chen Y. Minami E. Gill E.A. Ueno S. Yuan C. Gold J. Murry C.E. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015. - PubMed
-
- Shapiro A.M. Lakey J.R. Ryan E.A. Korbutt G.S. Toth E. Warnock G.L. Kneteman N.M. Rajotte R.V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230. - PubMed
-
- Rafii S. Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702. - PubMed
-
- Bliss T. Guzman R. Daadi M. Steinberg G.K. Cell transplantation therapy for stroke. Stroke. 2007;38:817. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

