Combined mutation screening of NKX2-5, GATA4, and TBX5 in congenital heart disease: multiple heterozygosity and novel mutations
- PMID: 22011241
- PMCID: PMC3370385
- DOI: 10.1111/j.1747-0803.2011.00573.x
Combined mutation screening of NKX2-5, GATA4, and TBX5 in congenital heart disease: multiple heterozygosity and novel mutations
Abstract
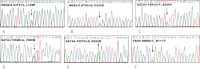
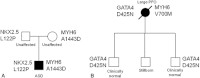
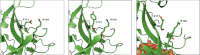
Background. Variants of several genes encoding transcription modulators, signal transduction, and structural proteins are known to cause Mendelian congenital heart disease (CHD). NKX2-5 and GATA4 were the first CHD-causing genes identified by linkage analysis in large affected families. Mutations of TBX5 cause Holt-Oram syndrome, which includes CHD as a clinical feature. All three genes have a well-established role in cardiac development. Design. In order to investigate the possible role of multiple mutations in CHD, a combined mutation screening was performed in NKX2-5, GATA4, and TBX5 in the same patient cohort. Samples from a cohort of 331 CHD patients were analyzed by polymerase chain reaction, double high-performance liquid chromatography and sequencing in order to identify changes in the NKX2-5, GATA4, and TBX5 genes. Results. Two cases of multiple heterozygosity of putative disease-causing mutations were identified. One patient was found with a novel L122P NKX2-5 mutation in combination with the private A1443D mutation of MYH6. A patient heterozygote for a D425N GATA4 mutation carries also a private mutation of the MYH6 gene (V700M). Conclusions. In addition to reporting two novel mutations of NKX2-5 in CHD, we describe families where multiple individual mutations seem to have an additive effect over the pathogenesis of CHD. Our findings highlight the usefulness of multiple gene mutational analysis of large CHD cohorts.
© 2011 Wiley Periodicals, Inc.
Figures


References
-
- Ferencz C. Epidemiology of Congenital Heart Disease: The Baltimore-Washington Infant Study 1981–1989. Mount Kisco: Futura Publishings; 1993.
-
- Burn J. The aetiology of congenital heart disease. In: Anderson RH, Baker EJ, Macartney FJ, Rigby ML, Shinebourne EA, Tynan M, editors. Paedriatic Cardiology. London: Churchill Livingstone; 2002. pp. 141–165.
-
- Nora JJ. Causes of congenital heart diseases: old and new modes, mechanisms, and models. Am Heart J. 1993;125:1409–1419. - PubMed
-
- Pierpont ME, Basson CT, Benson DW, et al. Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation. 2007;115:3015–3038. - PubMed
-
- Garg V, Kathiriya IS, Barnes R, et al. GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5. Nature. 2003;424:443–447. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

