Oxy intermediates of homoprotocatechuate 2,3-dioxygenase: facile electron transfer between substrates
- PMID: 22011290
- PMCID: PMC3222778
- DOI: 10.1021/bi201436n
Oxy intermediates of homoprotocatechuate 2,3-dioxygenase: facile electron transfer between substrates
Abstract
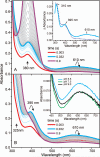
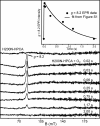
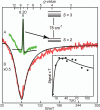
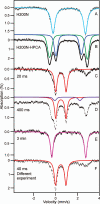
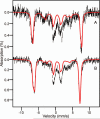
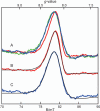
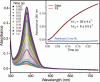
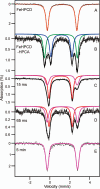
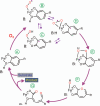
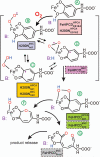
Substrates homoprotocatechuate (HPCA) and O(2) bind to the Fe(II) of homoprotocatechuate 2,3-dioxygenase (FeHPCD) in adjacent coordination sites. Transfer of an electron(s) from HPCA to O(2) via the iron is proposed to activate the substrates for reaction with each other to initiate aromatic ring cleavage. Here, rapid-freeze-quench methods are used to trap and spectroscopically characterize intermediates in the reactions of the HPCA complexes of FeHPCD and the variant His200Asn (FeHPCD−HPCA and H200N−HPCA, respectively) with O(2). A blue intermediate forms within 20 ms of mixing of O(2) with H200N−HPCA (H200N(Int1)(HPCA)). Parallel mode electron paramagnetic resonance and Mössbauer spectroscopies show that this intermediate contains high-spin Fe(III) (S = 5/2) antiferromagnetically coupled to a radical (S(R) = 1/2) to yield an S = 2 state. Together, optical and Mössbauer spectra of the intermediate support assignment of the radical as an HPCA semiquinone, implying that oxygen is bound as a (hydro)peroxo ligand. H200N(Int1)(HPCA) decays over the next 2 s, possibly through an Fe(II) intermediate (H200N(Int2)(HPCA)), to yield the product and the resting Fe(II) enzyme. Reaction of FeHPCD−HPCA with O(2) results in rapid formation of a colorless Fe(II) intermediate (FeHPCD(Int1)(HPCA)). This species decays within 1 s to yield the product and the resting enzyme. The absence of a chromophore from a semiquinone or evidence of a spin-coupled species in FeHPCD(Int1)(HPCA) suggests it is an intermediate occurring after O(2) activation and attack. The similar Mössbauer parameters for FeHPCD(Int1)(HPCA) and H200N(Int2)(HPCA) suggest these are similar intermediates. The results show that transfer of an electron from the substrate to the O(2) via the iron does occur, leading to aromatic ring cleavage.
Figures
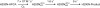
Similar articles
-
Ring-cleaving dioxygenases with a cupin fold.Appl Environ Microbiol. 2012 Apr;78(8):2505-14. doi: 10.1128/AEM.07651-11. Epub 2012 Jan 27. Appl Environ Microbiol. 2012. PMID: 22287012 Free PMC article. Review.
-
Substrate-mediated oxygen activation by homoprotocatechuate 2,3-dioxygenase: intermediates formed by a tyrosine 257 variant.Biochemistry. 2012 Nov 6;51(44):8743-54. doi: 10.1021/bi301114x. Epub 2012 Oct 29. Biochemistry. 2012. PMID: 23066705 Free PMC article.
-
NO binding to Mn-substituted homoprotocatechuate 2,3-dioxygenase: relationship to O₂ reactivity.J Biol Inorg Chem. 2013 Oct;18(7):717-28. doi: 10.1007/s00775-013-1016-2. Epub 2013 Jul 4. J Biol Inorg Chem. 2013. PMID: 23824380 Free PMC article.
-
A Long-Lived Fe(III)-(Hydroperoxo) Intermediate in the Active H200C Variant of Homoprotocatechuate 2,3-Dioxygenase: Characterization by Mössbauer, Electron Paramagnetic Resonance, and Density Functional Theory Methods.Inorg Chem. 2015 Nov 2;54(21):10269-80. doi: 10.1021/acs.inorgchem.5b01576. Epub 2015 Oct 20. Inorg Chem. 2015. PMID: 26485328 Free PMC article.
-
The ins and outs of ring-cleaving dioxygenases.Crit Rev Biochem Mol Biol. 2006 Jul-Aug;41(4):241-67. doi: 10.1080/10409230600817422. Crit Rev Biochem Mol Biol. 2006. PMID: 16849108 Review.
Cited by
-
Synthetic, spectroscopic, and DFT studies of iron complexes with iminobenzo(semi)quinone ligands: implications for o-aminophenol dioxygenases.Chemistry. 2013 Jul 15;19(29):9686-98. doi: 10.1002/chem.201300520. Epub 2013 Jun 6. Chemistry. 2013. PMID: 23744733 Free PMC article.
-
Ring-cleaving dioxygenases with a cupin fold.Appl Environ Microbiol. 2012 Apr;78(8):2505-14. doi: 10.1128/AEM.07651-11. Epub 2012 Jan 27. Appl Environ Microbiol. 2012. PMID: 22287012 Free PMC article. Review.
-
Rate-Determining Attack on Substrate Precedes Rieske Cluster Oxidation during Cis-Dihydroxylation by Benzoate Dioxygenase.Biochemistry. 2015 Aug 4;54(30):4652-64. doi: 10.1021/acs.biochem.5b00573. Epub 2015 Jul 21. Biochemistry. 2015. PMID: 26154836 Free PMC article.
-
Direct detection and characterization of bioinorganic peroxo moieties in a vanadium complex by 17O solid-state NMR and density functional theory.Solid State Nucl Magn Reson. 2018 Jul;91:15-20. doi: 10.1016/j.ssnmr.2018.02.001. Epub 2018 Feb 19. Solid State Nucl Magn Reson. 2018. PMID: 29506770 Free PMC article.
-
Nuclear Resonance Vibrational Spectroscopy Definition of Peroxy Intermediates in Catechol Dioxygenases: Factors that Determine Extra- versus Intradiol Cleavage.J Am Chem Soc. 2023 Jul 19;145(28):15230-15250. doi: 10.1021/jacs.3c02242. Epub 2023 Jul 6. J Am Chem Soc. 2023. PMID: 37414058 Free PMC article.
References
-
- Lipscomb JD, Orville AM. Mechanistic aspects of dihydroxybenzoate dioxygenases. Metal Ions Biol. Syst. 1992;28:243–298.
-
- Arciero DM, Lipscomb JD, Huynh BH, Kent TA, Münck E. EPR and Mössbauer studies of protocatechuate 4,5-dioxygenase. Characterization of a new Fe2+ environment. J. Biol. Chem. 1983;258:14981–14991. - PubMed
-
- Vaillancourt FH, Bolin JT, Eltis LD. The ins and outs of ring-cleaving dioxygenases. Crit. Rev. Biochem. Mol. Biol. 2006;41:241–267. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

