Spata22, a novel vertebrate-specific gene, is required for meiotic progress in mouse germ cells
- PMID: 22011390
- PMCID: PMC3290669
- DOI: 10.1095/biolreprod.111.095752
Spata22, a novel vertebrate-specific gene, is required for meiotic progress in mouse germ cells
Abstract
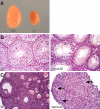
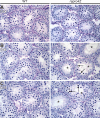
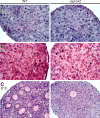
The N-ethyl-N-nitrosourea-induced repro42 mutation, identified by a forward genetics strategy, causes both male and female infertility, with no other apparent phenotypes. Positional cloning led to the discovery of a nonsense mutation in Spata22, a hitherto uncharacterized gene conserved among bony vertebrates. Expression of both transcript and protein is restricted predominantly to germ cells of both sexes. Germ cells of repro42 mutant mice express Spata22 transcript, but not SPATA22 protein. Gametogenesis is profoundly affected by the mutation, and germ cells in repro42 mutant mice do not progress beyond early meiotic prophase, with subsequent germ cell loss in both males and females. The Spata22 gene is essential for one or more key events of early meiotic prophase, as homologous chromosomes of mutant germ cells do not achieve normal synapsis or repair meiotic DNA double-strand breaks. The repro42 mutation thus identifies a novel mammalian germ cell-specific gene required for meiotic progression.
Figures



Comment in
-
Meiotic genetics moves forward with SPATA22 (repro42).Biol Reprod. 2012 Feb 29;86(2):42. doi: 10.1095/biolreprod.111.097436. Print 2012 Feb. Biol Reprod. 2012. PMID: 22088917 Free PMC article.
Similar articles
-
Meiotic genetics moves forward with SPATA22 (repro42).Biol Reprod. 2012 Feb 29;86(2):42. doi: 10.1095/biolreprod.111.097436. Print 2012 Feb. Biol Reprod. 2012. PMID: 22088917 Free PMC article.
-
Spermatogenesis associated 22 is required for DNA repair and synapsis of homologous chromosomes in mouse germ cells.Andrology. 2017 Mar;5(2):299-312. doi: 10.1111/andr.12315. Andrology. 2017. PMID: 28297563 Free PMC article.
-
Infertility associated with meiotic failure in the tremor rat (tm/tm) is caused by the deletion of spermatogenesis associated 22.Exp Anim. 2013;62(3):219-27. doi: 10.1538/expanim.62.219. Exp Anim. 2013. PMID: 23903057 Free PMC article.
-
Monitoring meiosis in gametogenesis.Theriogenology. 1998 Jan 15;49(2):423-30. doi: 10.1016/s0093-691x(97)00414-7. Theriogenology. 1998. PMID: 10732023 Review.
-
Sex matters in meiosis.Science. 2002 Jun 21;296(5576):2181-3. doi: 10.1126/science.1071907. Science. 2002. PMID: 12077403 Review.
Cited by
-
Integrated transcriptome analysis of mouse spermatogenesis.BMC Genomics. 2014 Jan 18;15:39. doi: 10.1186/1471-2164-15-39. BMC Genomics. 2014. PMID: 24438502 Free PMC article.
-
Meiotic genetics moves forward with SPATA22 (repro42).Biol Reprod. 2012 Feb 29;86(2):42. doi: 10.1095/biolreprod.111.097436. Print 2012 Feb. Biol Reprod. 2012. PMID: 22088917 Free PMC article.
-
Nuclear localization of human MEIOB requires its NLS in the OB domain and interaction with SPATA22.Acta Biochim Biophys Sin (Shanghai). 2022 Nov 25;55(1):154-161. doi: 10.3724/abbs.2022156. Acta Biochim Biophys Sin (Shanghai). 2022. PMID: 36331299 Free PMC article.
-
Hcn1 is a tremorgenic genetic component in a rat model of essential tremor.PLoS One. 2015 May 13;10(5):e0123529. doi: 10.1371/journal.pone.0123529. eCollection 2015. PLoS One. 2015. PMID: 25970616 Free PMC article.
-
Divergence and conservation of the meiotic recombination machinery.Nat Rev Genet. 2024 May;25(5):309-325. doi: 10.1038/s41576-023-00669-8. Epub 2023 Nov 30. Nat Rev Genet. 2024. PMID: 38036793 Review.
References
-
- Costa Y, Cooke HJ. Dissecting the mammalian synaptonemal complex using targeted mutations. Chromosome Res 2007; 15: 579 589 - PubMed
-
- de Boer E, Heyting C. The diverse roles of transverse filaments of synaptonemal complexes in meiosis. Chromosoma 2006; 115: 220 234 - PubMed
-
- Page SL, Hawley RS. The genetics and molecular biology of the synaptonemal complex. Annu Rev Cell Dev Biol 2004; 20: 525 558 - PubMed
-
- Cohen PE, Pollack SE, Pollard JW. Genetic analysis of chromosome pairing, recombination, and cell cycle control during first meiotic prophase in mammals. Endocr Rev 2006; 27: 398 426 - PubMed

