Release of metal ions from orthodontic appliances: an in vitro study
- PMID: 22011837
- PMCID: PMC3310133
- DOI: 10.1007/s12011-011-9233-4
Release of metal ions from orthodontic appliances: an in vitro study
Abstract
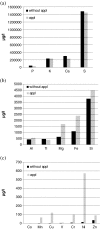
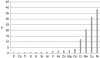
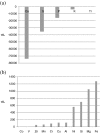
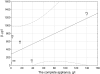
In this paper, we report the results of an in vitro experiment on the release of metal ions from orthodontic appliances composed of alloys containing iron, chromium, nickel, silicon, and molybdenum into artificial saliva. The concentrations of magnesium, aluminum, silicon, phosphorus, sulfur, potassium, calcium, titanium, vanadium, manganese, iron, cobalt, copper, zinc, nickel, and chromium were significantly higher in artificial saliva in which metal brackets, bands, and wires used in orthodontics were incubated. In relation to the maximum acceptable concentrations of metal ions in drinking water and to recommended daily doses, two elements of concern were nickel (573 vs. 15 μg/l in the controls) and chromium (101 vs. 8 μg/l in the controls). Three ion release coefficients were defined: α, a dimensionless multiplication factor; β, the difference in concentrations (in micrograms per liter); and γ, the ion release coefficient (in percent). The elevated levels of metals in saliva are thought to occur by corrosion of the chemical elements in the alloys or welding materials. The concentrations of some groups of dissolved elements appear to be interrelated.
Figures

References
-
- Wise DL (ed) (2000) Biomaterials and bioengineering handbook. Marcel Dekker, New York
-
- Pereira ML, Silva A, Tracana R, Carvalho GS. Toxic effects caused by stainless steel corrosion products on mouse seminiferous cells. Cytobios. 1994;77:73–80. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

