Identification and characterization of tumor-initiating cells in human primary cutaneous squamous cell carcinoma
- PMID: 22011906
- PMCID: PMC3258300
- DOI: 10.1038/jid.2011.317
Identification and characterization of tumor-initiating cells in human primary cutaneous squamous cell carcinoma
Abstract
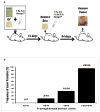
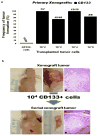
Primary human squamous cell carcinomas (SCCas) are heterogeneous invasive tumors with proliferating outer layers and inner differentiating cell masses. To determine if tumor-initiating cells (TICs) are present in SCCas, we utilized newly developed reliable in vitro and in vivo xenograft assays that propagate human SCCas, and demonstrated that a small subset of SCCa cells (∼1%) expressing Prominin-1 (CD133) in the outer layers of SCCas were highly enriched for TICs (∼1/400) compared with unsorted SCCa cells (TICs ∼1/10(6)). Xenografts of CD133+ SCCas recreated the original SCCa tumor histology and organizational hierarchy, whereas CD133- cells did not, and only CD133+ cells demonstrated the capacity for self-renewal in serial transplantation studies. We present a model of human SCCas in which tumor projections expand with outer leading edges that contain CD133+ TICs. Successful cancer treatment will likely require that the TICs identified in cancers be targeted therapeutically. The demonstration that TICs are present in SCCas and are enriched in a CD133- expressing subpopulation has not been, to our knowledge, previously reported.
Figures









Comment in
-
A new xenotransplantation model reveals tumor-initiating cells in cutaneous squamous cell carcinoma.J Invest Dermatol. 2012 Feb;132(2):261-2. doi: 10.1038/jid.2011.327. J Invest Dermatol. 2012. PMID: 22241440
Similar articles
-
Identification of Human Cutaneous Squamous Cell Carcinoma Cancer Stem Cells.Methods Mol Biol. 2019;1879:415-433. doi: 10.1007/7651_2018_134. Methods Mol Biol. 2019. PMID: 29582375
-
Combination Treatment with All-Trans Retinoic Acid Prevents Cisplatin-Induced Enrichment of CD133+ Tumor-Initiating Cells and Reveals Heterogeneity of Cancer Stem Cell Compartment in Lung Cancer.J Thorac Oncol. 2015 Jul;10(7):1027-36. doi: 10.1097/JTO.0000000000000563. J Thorac Oncol. 2015. PMID: 26020124 Free PMC article.
-
ALDH activity selectively defines an enhanced tumor-initiating cell population relative to CD133 expression in human pancreatic adenocarcinoma.PLoS One. 2011;6(6):e20636. doi: 10.1371/journal.pone.0020636. Epub 2011 Jun 13. PLoS One. 2011. PMID: 21695188 Free PMC article.
-
CD133(+) cells isolated from various sources and their role in future clinical perspectives.Expert Opin Biol Ther. 2010 Nov;10(11):1521-8. doi: 10.1517/14712598.2010.528386. Epub 2010 Oct 11. Expert Opin Biol Ther. 2010. PMID: 20932225 Review.
-
CD133: a cancer stem cells marker, is used in colorectal cancers.World J Gastroenterol. 2013 May 7;19(17):2603-11. doi: 10.3748/wjg.v19.i17.2603. World J Gastroenterol. 2013. PMID: 23674867 Free PMC article. Review.
Cited by
-
Cancer Stem Cells in Squamous Cell Carcinoma.J Invest Dermatol. 2017 Jan;137(1):31-37. doi: 10.1016/j.jid.2016.07.033. Epub 2016 Nov 24. J Invest Dermatol. 2017. PMID: 27638386 Free PMC article. Review.
-
Biochemistry of epidermal stem cells.Biochim Biophys Acta. 2013 Feb;1830(2):2427-34. doi: 10.1016/j.bbagen.2012.07.002. Epub 2012 Jul 20. Biochim Biophys Acta. 2013. PMID: 22820019 Free PMC article. Review.
-
Correlation of Ki-67 Expression as Tumor cell Proliferation Activity Marker with Cutaneous Squamous Cell Carcinoma Grading.Open Access Maced J Med Sci. 2019 Oct 14;7(20):3384-3386. doi: 10.3889/oamjms.2019.428. eCollection 2019 Oct 30. Open Access Maced J Med Sci. 2019. PMID: 32002056 Free PMC article.
-
CD200-expressing human basal cell carcinoma cells initiate tumor growth.Proc Natl Acad Sci U S A. 2013 Jan 22;110(4):1434-9. doi: 10.1073/pnas.1211655110. Epub 2013 Jan 4. Proc Natl Acad Sci U S A. 2013. PMID: 23292936 Free PMC article.
-
Donor-derived stem-cells and epithelial mesenchymal transition in squamous cell carcinoma in transplant recipients.Oncotarget. 2015 Dec 8;6(39):41497-507. doi: 10.18632/oncotarget.6359. Oncotarget. 2015. PMID: 26594799 Free PMC article.
References
-
- Benton EC, Kerr OA, Fisher A, Fraser SJ, McCormack SKA, Tidman MJ. The changing face of dermatological practice: 25 years’ experience. Br J Dermatol. 2008;159:413–418. - PubMed
-
- Boukamp P. Non-melanoma skin cancer: what drives tumor development and progression? Carcinogenesis. 2005;26:1657–1667. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

