Differential activation of RAGE by HMGB1 modulates neutrophil-associated NADPH oxidase activity and bacterial killing
- PMID: 22012330
- PMCID: PMC3328912
- DOI: 10.1152/ajpcell.00302.2011
Differential activation of RAGE by HMGB1 modulates neutrophil-associated NADPH oxidase activity and bacterial killing
Abstract
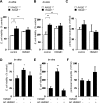
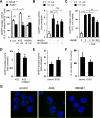
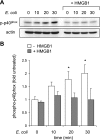
The receptor for advanced glycation end products (RAGE) plays an important role in host defense against bacterial infection. In the present experiments, we investigated the mechanisms by which RAGE contributes to the ability of neutrophils to eradicate bacteria. Wild-type (RAGE(+/+)) neutrophils demonstrated significantly greater ability to kill Escherichia coli compared with RAGE(-/-) neutrophils. After intraperitoneal injection of E. coli, increased numbers of bacteria were found in the peritoneal fluid from RAGE(-/-) as compared with RAGE(+/+) mice. Exposure of neutrophils to the protypical RAGE ligand AGE resulted in activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and enhanced killing of E. coli, and intraperitoneal injection of AGE enhanced bacterial clearance during peritonitis. However, incubation of neutrophils with high mobility group box 1 protein (HMGB1), which also binds to RAGE, diminished E. coli-induced activation of NADPH oxidase in neutrophils and bacterial killing both in vitro and in vivo. Deletion of the COOH-terminal tail of HMGB1, a region necessary for binding to RAGE, abrogated the ability of HMGB1 to inhibit bacterial killing. Incubation of neutrophils with HMGB1 diminished bacterial or AGE-dependent activation of NADPH oxidase. The increase in phosphorylation of the p40(phox) subunit of NADPH oxidase that occurred after culture of neutrophils with E. coli was inhibited by exposure of the cells to HMGB1. These results showing that HMGB1, through RAGE-dependent mechanisms, diminishes bacterial killing by neutrophils as well as NADPH oxidase activation provide a novel mechanism by which HMGB1 can potentiate sepsis-associated organ dysfunction and mortality.
Figures
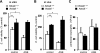

Similar articles
-
HMGB1-RAGE pathway drives peroxynitrite signaling-induced IBD-like inflammation in murine nonalcoholic fatty liver disease.Redox Biol. 2017 Oct;13:8-19. doi: 10.1016/j.redox.2017.05.005. Epub 2017 May 10. Redox Biol. 2017. PMID: 28551086 Free PMC article.
-
Hemorrhagic shock activates lung endothelial reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase via neutrophil NADPH oxidase.Am J Respir Cell Mol Biol. 2011 Mar;44(3):333-40. doi: 10.1165/rcmb.2009-0408OC. Epub 2010 Apr 23. Am J Respir Cell Mol Biol. 2011. PMID: 20418360 Free PMC article.
-
Frontline Science: HMGB1 induces neutrophil dysfunction in experimental sepsis and in patients who survive septic shock.J Leukoc Biol. 2017 Jun;101(6):1281-1287. doi: 10.1189/jlb.5HI0316-128RR. Epub 2016 Dec 13. J Leukoc Biol. 2017. PMID: 27965385 Free PMC article.
-
NADPH oxidase activation in neutrophils: Role of the phosphorylation of its subunits.Eur J Clin Invest. 2018 Nov;48 Suppl 2:e12951. doi: 10.1111/eci.12951. Epub 2018 Jun 3. Eur J Clin Invest. 2018. PMID: 29757466 Review.
-
Expatiating the molecular approaches of HMGB1 in diabetes mellitus: Highlighting signalling pathways via RAGE and TLRs.Mol Biol Rep. 2021 Feb;48(2):1869-1881. doi: 10.1007/s11033-020-06130-x. Epub 2021 Jan 21. Mol Biol Rep. 2021. PMID: 33479829 Review.
Cited by
-
HMGB1 in health and disease.Mol Aspects Med. 2014 Dec;40:1-116. doi: 10.1016/j.mam.2014.05.001. Epub 2014 Jul 8. Mol Aspects Med. 2014. PMID: 25010388 Free PMC article. Review.
-
High-mobility group box1 as an amplifier of immune response and target for treatment in Aspergillus fumigatus keratitis.Int J Ophthalmol. 2020 May 18;13(5):708-717. doi: 10.18240/ijo.2020.05.03. eCollection 2020. Int J Ophthalmol. 2020. PMID: 32420216 Free PMC article.
-
Pathophysiological Role of Neutrophil Extracellular Traps in Diet-Induced Obesity and Metabolic Syndrome in Animal Models.Nutrients. 2025 Jan 10;17(2):241. doi: 10.3390/nu17020241. Nutrients. 2025. PMID: 39861371 Free PMC article. Review.
-
Protumor and antitumor functions of neutrophil granulocytes.Semin Immunopathol. 2013 Mar;35(2):163-76. doi: 10.1007/s00281-012-0344-6. Epub 2012 Sep 25. Semin Immunopathol. 2013. PMID: 23007469 Review.
-
Role of heme in lung bacterial infection after trauma hemorrhage and stored red blood cell transfusion: A preclinical experimental study.PLoS Med. 2018 Mar 9;15(3):e1002522. doi: 10.1371/journal.pmed.1002522. eCollection 2018 Mar. PLoS Med. 2018. PMID: 29522519 Free PMC article.
References
-
- Angus DC, Yang L, Kong L, Kellum JA, Delude RL, Tracey KJ, Weissfeld L. Circulating high-mobility group box 1 (HMGB1) concentrations are elevated in both uncomplicated pneumonia and pneumonia with severe sepsis. Crit Care Med 35: 1061–1067, 2007 - PubMed
-
- Babior BM. NADPH oxidase: an update. Blood 93: 1464–1476, 1999 - PubMed
-
- Bierhaus A, Schiekofer S, Schwaninger M, Andrassy M, Humpert PM, Chen J, Hong M, Luther T, Henle T, Kloting I, Morcos M, Hofmann M, Tritschler H, Weigle B, Kasper M, Smith M, Perry G, Schmidt AM, Stern DM, Haring HU, Schleicher E, Nawroth PP. Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes 50: 2792–2808, 2001 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

