Activation of the STAT6 transcription factor in Jurkat T-cells by the herpesvirus saimiri Tip protein
- PMID: 22012462
- PMCID: PMC3352339
- DOI: 10.1099/vir.0.036087-0
Activation of the STAT6 transcription factor in Jurkat T-cells by the herpesvirus saimiri Tip protein
Abstract
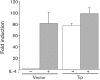
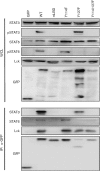
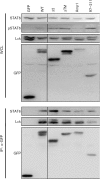
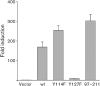
Herpesvirus saimiri (HVS), a T-lymphotropic monkey herpesvirus, induces fulminant T-cell lymphoma in non-natural primate hosts. In addition, it can immortalize human T-cells in vitro. HVS tyrosine kinase-interacting protein (Tip) is an essential viral gene required for T-cell transformation both in vitro and in vivo. In this study, we found that Tip interacts with the STAT6 transcription factor and induces phosphorylation of STAT6 in T-cells. The interaction with STAT6 requires the Tyr(127) residue and Lck-binding domain of Tip, which are indispensable for interleukin (IL)-2-independent T-cell transformation by HVS. It was also demonstrated that Tip induces nuclear translocation of STAT6, as well as activation of STAT6-dependent transcription in Jurkat T-cells. Interestingly, the phosphorylated STAT6 mainly colocalized with vesicles containing Tip within T-cells, but was barely detectable in the nucleus. However, nuclear translocation of phospho-STAT6 and transcriptional activation of STAT6 by IL-4 stimulation were not affected significantly in T-cells expressing Tip. Collectively, these findings suggest that the constitutive activation of STAT6 by Tip in T-cells may contribute to IL-2-independent T-cell transformation by HVS.
Figures



References
-
- Biesinger B., Tsygankov A. Y., Fickenscher H., Emmrich F., Fleckenstein B., Bolen J. B., Bröker B. M. (1995). The product of the Herpesvirus saimiri open reading frame 1 (Tip) interacts with T cell-specific kinase p56lck in transformed cells. J Biol Chem 270, 4729–4734 10.1074/jbc.270.9.4729 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

