Protonation sites and dissociation mechanisms of t-butylcarbamates in tandem mass spectrometric assays for newborn screening
- PMID: 22012676
- PMCID: PMC3212097
- DOI: 10.1002/jms.1993
Protonation sites and dissociation mechanisms of t-butylcarbamates in tandem mass spectrometric assays for newborn screening
Abstract
Structures of tert-butylcarbamate ions in the gas-phase and methanol solution were studied for simple secondary and tertiary carbamates as well as for carbamate-containing products and internal standards for lysosomal enzyme assays used in newborn screening of a α-galactosidase A deficiency (Fabry disease), mucopolysaccharidosis I (Hurler disease), and mucopolysaccharidosis II (Hunter disease). The protonation of simple t-butylcarbamates can occur at the carbonyl group, which is the preferred site in the gas phase. Protonation in methanol solution is more favorable if occurring at the carbamate nitrogen atom. The protonation of more complex t-butylcarbamates occurs at amide and carbamate carbonyl groups, and the ions are stabilized by intramolecular hydrogen bonding, which is affected by solvation. Tertiary carbamates containing aminophenol amide groups were calculated to have substantially greater gas-phase basicities than secondary carbamates containing coumarin amide groups. The main diagnostically important ion dissociation by elimination of 2-methylpropene (isobutylene, i-C(4)H(8)) and carbon dioxide is shown by experiment and theory to proceed in two steps. Energy-resolved collision-induced dissociation of the Hurler's disease enzymatic product ion, which is a coumarin-diamine linker-t-butylcarbamate conjugate (3a(+)), indicated separate energy thresholds for the loss of i-C(4)H(8) and CO(2). Computational investigation of the potential energy surface along two presumed reaction pathways indicated kinetic preference for the migration of a t-butyl hydrogen atom to the carbamate carbonyl resulting in the isobutylene loss. The consequent loss of CO(2) required further proton migrations that had to overcome energy barriers.
Copyright © 2011 John Wiley & Sons, Ltd.
Figures
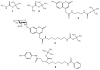




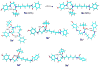




References
-
- Pearson AJ, Roush WR. Handbook of Reagents for Organic Synthesis - Activating Agents and Protecting Groups. John Wiley & Sons; 1999. p. 83. online version http://www.knovel.com/web/portal/browse/display?_EXT_KNOVEL_DISPLAY_book....
-
- Ashworth IW, Cox BG, Meyrick B. Kinetics and mechanism of N-Boc cleavage: Evidence of a second-order dependence upon acid concentration. J Org Chem. 2010;75:8117–8125. - PubMed
-
- Garner GV, Gordon DB, Tetler LW, Sedgwick RD. Fast atom bombardment mass spectrometry of butyloxycarbonyl protected (BOC) amino acids. Org Mass Spectrom. 1988;18:486–488.
-
- Tureček F, Scott CR, Gelb MH. Methods in Molecular Biology. Totowa, NJ, United States: 2007. Tandem mass spectrometry in the detection of inborn errors of metabolism for newborn screening; p. 359. - PubMed
- Quantitative Proteomics by Mass Spectrometry. pp. 143–157.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

