Twists and turns in ubiquitin-like protein conjugation cascades
- PMID: 22012881
- PMCID: PMC3302639
- DOI: 10.1002/pro.750
Twists and turns in ubiquitin-like protein conjugation cascades
Abstract
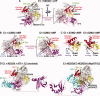
Post-translational modification by ubiquitin-like proteins (UBLs) is a predominant eukaryotic regulatory mechanism. The vast reach of this form of regulation extends to virtually all eukaryotic processes that involve proteins. UBL modifications play critical roles in controlling the cell cycle, transcription, DNA repair, stress responses, signaling, immunity, plant growth, embryogenesis, circadian rhythms, and a plethora of other pathways. UBLs dynamically modulate target protein properties including enzymatic activity, conformation, half-life, subcellular localization, and intermolecular interactions. Moreover, the enzymatic process of UBL ligation to proteins is itself dynamic, with the UBL moving between multiple enzyme active sites and ultimately to a target. This review highlights our work on how the dynamic conformations of selected enzymes catalyzing UBL ligation help mediate this fascinating form of protein regulation.
Copyright © 2011 The Protein Society.
Figures


References
-
- Hicke L. Protein regulation by monoubiquitin. Nat Rev. 2001;2:195–201. - PubMed
-
- Di Fiore PP, Polo S, Hofmann K. When ubiquitin meets ubiquitin receptors: a signalling connection. Nature reviews. Mol Cell Biol. 2003;4:491–497. - PubMed
-
- Hicke L, Schubert HL, Hill CP. Ubiquitin-binding domains. Nat Rev. 2005;6:610–621. - PubMed
-
- Morita E, Sundquist WI. Retrovirus budding. Ann Rev Cell Dev Biol. 2004;20:395–425. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

