Initial testing of the investigational NEDD8-activating enzyme inhibitor MLN4924 by the pediatric preclinical testing program
- PMID: 22012946
- PMCID: PMC3823062
- DOI: 10.1002/pbc.23357
Initial testing of the investigational NEDD8-activating enzyme inhibitor MLN4924 by the pediatric preclinical testing program
Erratum in
- Pediatr Blood Cancer. 2012 Oct;59(4):772
Abstract
Background: MLN4924 is an investigational first-in-class small molecule inhibitor of NEDD8-activating enzyme (NAE). NAE is an essential component of the NEDD8 conjugation pathway, controlling the activity of a subset of ubiquitin-proteasome system (UPS) E3 ligases, multiprotein complexes that transfer ubiquitin molecules to substrate proteins.
Procedures: MLN4924 was tested against the PPTP in vitro panel using 96-hour exposure time at concentrations ranging from 1.0 nM to 10 µM. It was tested in vivo at a dose of 100 mg/kg [66 mg/kg for the acute lymphoblastic leukemia (ALL) xenografts] administered orally twice daily × 5 days. Treatment duration was 3 weeks.
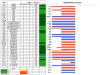
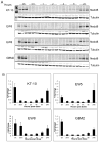
Results: The median relative IC(50) for MLN4924 against the PPTP cell lines was 143 nM, (range: 15-678 nM) with that for the Ewing panel being significantly lower (31 nM). MLN4924 induced significant differences in EFS distribution compared to control in 20 of 34 (59%) evaluable solid tumor xenografts. MLN4924 induced intermediate activity (EFS T/C values >2) in 9 of the 33 evaluable xenografts (27%), including 4 of 4 glioblastoma xenografts, 2 of 3 Wilm's tumor xenografts, 2 of 5 rhabdomyosarcoma xenografts, and 1 of 4 neuroblastoma xenografts. For the ALL panel, 5 of 8 evaluable xenografts showed intermediate activity for the EFS T/C measure. MLN4924 did not induce objective responses in the PPTP solid tumor or ALL panels.
Conclusions: MLN4924 showed potent activity in vitro and in vivo showed tumor growth inhibitory activity against a subset of the PPTP solid tumor and ALL xenografts.
Copyright © 2011 Wiley Periodicals, Inc.
Conflict of interest statement
Figures


References
-
- Brownell JE, Sintchak MD, Gavin JM, et al. Substrate-assisted inhibition of ubiquitin-like protein-activating enzymes: the NEDD8 E1 inhibitor MLN4924 forms a NEDD8-AMP mimetic in situ. Molecular cell. 2010;37(1):102–111. - PubMed
-
- Milhollen MA, Narayanan U, Soucy TA, et al. Inhibition of NEDD8-activating enzyme induces rereplication and apoptosis in human tumor cells consistent with deregulating CDT1 turnover. Cancer research. 2011;71(8):3042–3051. - PubMed
-
- Soucy TA, Smith PG, Milhollen MA, et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. 2009;458(7239):732–736. - PubMed
-
- Milhollen MA, Traore T, Adams-Duffy J, et al. MLN4924, a NEDD8-activating enzyme inhibitor, is active in diffuse large B-cell lymphoma models: rationale for treatment of NF-{kappa}B-dependent lymphoma. Blood. 2010 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

