The vaccinia virus-encoded Bcl-2 homologues do not act as direct Bax inhibitors
- PMID: 22013032
- PMCID: PMC3255923
- DOI: 10.1128/JVI.05817-11
The vaccinia virus-encoded Bcl-2 homologues do not act as direct Bax inhibitors
Abstract
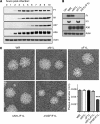
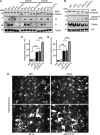
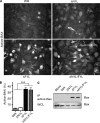
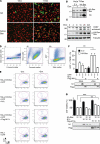
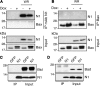
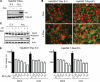
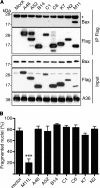
Many viruses, including members of several poxvirus genera, encode inhibitors that block apoptosis by simultaneously binding the proapoptotic Bcl-2 proteins Bak and Bax. The Orthopoxvirus vaccinia virus encodes the Bcl-2-like F1 protein, which sequesters Bak but not Bax. However, N1, a potent virulence factor, is reported to be antiapoptotic and to interact with Bax. Here we investigated whether vaccinia virus inhibits Bak/Bax-dependent apoptosis via the cooperative action of F1 and N1. We found that Western Reserve (WR) and ΔN1L viruses inhibited drug- and infection-induced apoptosis equally. Meanwhile, infections with ΔF1L or ΔN1L/F1L virus resulted in similar levels of Bax activation and apoptosis. Outside the context of infection, N1 did not block drug- or Bax-induced cell death or interact with Bax. In addition to F1 and N1, vaccinia virus encodes further structural homologs of Bcl-2 proteins that are conserved in orthopoxviruses, including A46, A52, B14, C1, C6, C16/B22, K7, and N2. However, we found that these do not associate with Bax or inhibit drug-induced cell death. Based on our findings that N1 is not an antiapoptotic protein, we propose that the F1 orthologs represent the only orthopoxvirus Bcl-2 homolog to directly inhibit the Bak/Bax checkpoint.
Figures
References
-
- Arakawa Y, Cordeiro JV, Way M. 2007. F11L-mediated inhibition of RhoA-mDia signaling stimulates microtubule dynamics during vaccinia virus infection. Cell Host Microbe 1:213–226 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

