Secretion modification region-derived peptide disrupts HIV-1 Nef's interaction with mortalin and blocks virus and Nef exosome release
- PMID: 22013042
- PMCID: PMC3255900
- DOI: 10.1128/JVI.05720-11
Secretion modification region-derived peptide disrupts HIV-1 Nef's interaction with mortalin and blocks virus and Nef exosome release
Abstract
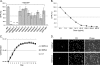
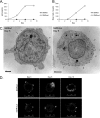
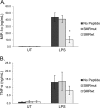
Nef is secreted from infected cells in exosomes and is found in abundance in the sera of HIV-infected individuals. Secreted exosomal Nef (exNef) induces apoptosis in uninfected CD4⁺ T cells and may be a key component of HIV pathogenesis. The exosomal pathway has been implicated in HIV-1 virus release, suggesting a possible link between these two viral processes. However, the underlying mechanisms and cellular components of exNef secretion have not been elucidated. We have previously described a Nef motif, the secretion modification region (SMR; amino acids 66 to 70), that is required for exNef secretion. In silico modeling data suggest that this motif can form a putative binding pocket. We hypothesized that the Nef SMR binds a cellular protein involved in protein trafficking and that inhibition of this interaction would abrogate exNef secretion. By using tandem mass spectrometry and coimmunoprecipitation with a novel SMR-based peptide (SMRwt) that blocks exNef secretion and HIV-1 virus release, we identified mortalin as an SMR-specific cellular protein. A second set of coimmunoprecipitation experiments with full-length Nef confirmed that mortalin interacts with Nef via Nef's SMR motif and that this interaction is disrupted by the SMRwt peptide. Overexpression and microRNA knockdown of mortalin revealed a positive correlation between exNef secretion levels and mortalin protein expression. Using antibody inhibition we demonstrated that the Nef/mortalin interaction is necessary for exNef secretion. Taken together, this work constitutes a significant step in understanding the underlying mechanism of exNef secretion, identifies a novel host-pathogen interaction, and introduces an HIV-derived peptide with antiviral properties.
Figures



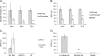
Similar articles
-
Peptide-based identification of functional motifs and their binding partners.J Vis Exp. 2013 Jun 30;(76):50362. doi: 10.3791/50362. J Vis Exp. 2013. PMID: 23852082 Free PMC article.
-
Hallmarks of HIV-1 pathogenesis are modulated by Nef's Secretion Modification Region.J AIDS Clin Res. 2015 Jul;6(7):476. doi: 10.4172/2155-6113.1000476. Epub 2015 Jun 30. J AIDS Clin Res. 2015. PMID: 26523240 Free PMC article.
-
Microglia-derived HIV Nef+ exosome impairment of the blood-brain barrier is treatable by nanomedicine-based delivery of Nef peptides.J Neurovirol. 2016 Apr;22(2):129-39. doi: 10.1007/s13365-015-0397-0. Epub 2015 Dec 2. J Neurovirol. 2016. PMID: 26631079
-
How HIV Nef Proteins Hijack Membrane Traffic To Promote Infection.J Virol. 2019 Nov 26;93(24):e01322-19. doi: 10.1128/JVI.01322-19. Print 2019 Dec 15. J Virol. 2019. PMID: 31578291 Free PMC article. Review.
-
Mortalin - a multipotent chaperone regulating cellular processes ranging from viral infection to neurodegeneration.Acta Virol. 2013;57(1):3-15. doi: 10.4149/av_2013_01_3. Acta Virol. 2013. PMID: 23530819 Review.
Cited by
-
Overlapping effector interfaces define the multiple functions of the HIV-1 Nef polyproline helix.Retrovirology. 2012 May 31;9:47. doi: 10.1186/1742-4690-9-47. Retrovirology. 2012. PMID: 22651890 Free PMC article.
-
Exosomal MicroRNA-155 Inhibits Enterovirus A71 Infection by Targeting PICALM.Int J Biol Sci. 2019 Nov 15;15(13):2925-2935. doi: 10.7150/ijbs.36388. eCollection 2019. Int J Biol Sci. 2019. PMID: 31853228 Free PMC article.
-
HIV-1 Nef in macrophage-mediated disease pathogenesis.Int Rev Immunol. 2012 Dec;31(6):432-50. doi: 10.3109/08830185.2012.737073. Int Rev Immunol. 2012. PMID: 23215766 Free PMC article. Review.
-
HIV Neuroinflammation: The Role of Exosomes in Cell Signaling, Prognostic and Diagnostic Biomarkers and Drug Delivery.Front Cell Dev Biol. 2021 Apr 1;9:637192. doi: 10.3389/fcell.2021.637192. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33869183 Free PMC article. Review.
-
Brain transcriptome-wide screen for HIV-1 Nef protein interaction partners reveals various membrane-associated proteins.PLoS One. 2012;7(12):e51578. doi: 10.1371/journal.pone.0051578. Epub 2012 Dec 17. PLoS One. 2012. PMID: 23284715 Free PMC article.
References
-
- Aiken C, Konner J, Landau NR, Lenburg ME, Trono D. 1994. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell 76:853–864 - PubMed
-
- Annunziata P. 2003. Blood-brain barrier changes during invasion of the central nervous system by HIV-1. Old and new insights into the mechanism. J. Neurol. 250:901–906 - PubMed
-
- Bagasra O, Hauptman SP, Lischner HW, Sachs M, Pomerantz RJ. 1992. Detection of human immunodeficiency virus type 1 provirus in mononuclear cells by in situ polymerase chain reaction. N. Engl. J. Med. 326:1385–1391 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 A1050409/PHS HHS/United States
- S06 GM08248/GM/NIGMS NIH HHS/United States
- U54 MD007588/MD/NIMHD NIH HHS/United States
- G12 RR003034/RR/NCRR NIH HHS/United States
- C06 RR018386/RR/NCRR NIH HHS/United States
- C06 RR18386/RR/NCRR NIH HHS/United States
- G12-RR03034/RR/NCRR NIH HHS/United States
- F31 AI091484/AI/NIAID NIH HHS/United States
- S21 MD000101/MD/NIMHD NIH HHS/United States
- S06 GM008248/GM/NIGMS NIH HHS/United States
- R25 GM058268/GM/NIGMS NIH HHS/United States
- F31AI091484/AI/NIAID NIH HHS/United States
- U54 RR026137/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

