Mutations at the base of the icosahedral five-fold cylinders of minute virus of mice induce 3'-to-5' genome uncoating and critically impair entry functions
- PMID: 22013064
- PMCID: PMC3255873
- DOI: 10.1128/JVI.06119-11
Mutations at the base of the icosahedral five-fold cylinders of minute virus of mice induce 3'-to-5' genome uncoating and critically impair entry functions
Abstract
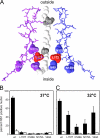
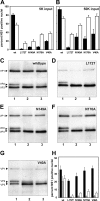
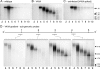
The linear single-stranded DNA genome of minute virus of mice can be ejected, in a 3'-to-5' direction, via a cation-linked uncoating reaction that leaves the 5' end of the DNA firmly complexed with its otherwise intact protein capsid. Here we compare the phenotypes of four mutants, L172T, V40A, N149A, and N170A, which perturb the base of cylinders surrounding the icosahedral 5-fold axes of the virus, and show that these structures are strongly implicated in 3'-to-5' release. Although noninfectious at 37°C, all mutants were viable at 32°C, showed a temperature-sensitive cell entry defect, and, after proteolysis of externalized VP2 N termini, were unable to protect the VP1 domain, which is essential for bilayer penetration. Mutant virus yields from multiple-round infections were low and were characterized by the accumulation of virions containing subgenomic DNAs of specific sizes. In V40A, these derived exclusively from the 5' end of the genome, indicative of 3'-to-5' uncoating, while L172T, the most impaired mutant, had long subgenomic DNAs originating from both termini, suggesting additional packaging portal defects. Compared to the wild type, genome release in vitro following cation depletion was enhanced for all mutants, while only L172T released DNA, in both directions, without cation depletion following proteolysis at 37°C. Analysis of progeny from single-round infections showed that uncoating did not occur during virion assembly, release, or extraction. However, unlike the wild type, the V40A mutant extensively uncoated during cell entry, indicating that the V40-L172 interaction restrains an uncoating trigger mechanism within the endosomal compartment.
Figures





References
-
- Agbandje-McKenna M, Llamas-Saiz AL, Wang F, Tattersall P, Rossmann MG. 1998. Functional implications of the structure of the murine parvovirus, minute virus of mice. Structure 6:1369–1381 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

