Characterization of the Src/Abl hybrid kinase SmTK6 of Schistosoma mansoni
- PMID: 22013071
- PMCID: PMC3234968
- DOI: 10.1074/jbc.M110.210336
Characterization of the Src/Abl hybrid kinase SmTK6 of Schistosoma mansoni
Abstract
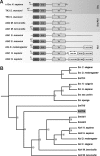
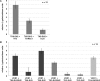
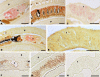
Cellular protein-tyrosine kinases play key roles in signal transduction processes in eukaryotes. SmTK4 was the first Syk kinase identified in a parasite and found to be tissue-specifically transcribed in the gonads of adult Schistosoma mansoni. Functional analyses confirmed its role in oogenesis and spermatogenesis. As an SmTK4 upstream binding partner, the cellular protein-tyrosine kinase SmTK6 was isolated from a yeast two-hybrid library. Phylogenetic analyses performed in this study confirmed the first suggestions of a hybrid character of SmTK6. Biochemical studies made in Xenopus oocytes using inhibitors against Src (herbimycin A) and Abl (imatinib) kinases exhibited a biochemical inhibition profile of SmTK6, which was intermediate of Src and Abl kinases. As SmTK6 upstream interaction partners, we identified among others the known Src kinase SmTK3 and the Venus kinase receptor SmVKR1 of S. mansoni by yeast two-hybrid analyses, all of which co-localized in the gonads. Co-immunoprecipitation experiments confirmed interactions between SmTK6 and SmTK3 or SmVKR1. In Xenopus oocytes, it was finally shown that SmVKR1 but also SmTK3 were able to activate SmTK6 enzymatic activity indicating its functions in a receptor tyrosine kinase signal transduction cascade. These results not only demonstrate an intermediate but Src-biased profile of the unusual kinase SmTK6. They also strongly substantiate previous indications for a kinase complex, consisting of a receptor tyrosine kinase, Syk and Src kinases, which has been hypothesized to be involved in proliferation and differentiation processes in the gonads of schistosomes.
Figures
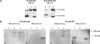
References
-
- Bromann P. A., Korkaya H., Courtneidge S. A. (2004) Oncogene 23, 7957–7968 - PubMed
-
- Takahashi A., Obata Y., Fukumoto Y., Nakayama Y., Kasahara K., Kuga T., Higashiyama Y., Saito T., Yokoyama K. K., Yamaguchi N. (2009) Exp. Cell Res. 315, 1117–1141 - PubMed
-
- Hubbard S. R., Till J. H. (2000) Annu. Rev. Biochem. 69, 373–398 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

