A novel combination immunotherapy for cancer by IL-13Rα2-targeted DNA vaccine and immunotoxin in murine tumor models
- PMID: 22013118
- PMCID: PMC3730529
- DOI: 10.4049/jimmunol.1102095
A novel combination immunotherapy for cancer by IL-13Rα2-targeted DNA vaccine and immunotoxin in murine tumor models
Abstract
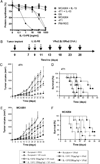
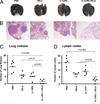
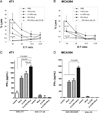
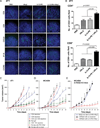
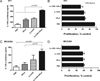
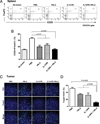
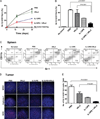
Optimum efficacy of therapeutic cancer vaccines may require combinations that generate effective antitumor immune responses, as well as overcome immune evasion and tolerance mechanisms mediated by progressing tumor. Previous studies showed that IL-13Rα2, a unique tumor-associated Ag, is a promising target for cancer immunotherapy. A targeted cytotoxin composed of IL-13 and mutated Pseudomonas exotoxin induced specific killing of IL-13Rα2(+) tumor cells. When combined with IL-13Rα2 DNA cancer vaccine, surprisingly, it mediated synergistic antitumor effects on tumor growth and metastasis in established murine breast carcinoma and sarcoma tumor models. The mechanism of synergistic activity involved direct killing of tumor cells and cell-mediated immune responses, as well as elimination of myeloid-derived suppressor cells and, consequently, regulatory T cells. These novel results provide a strong rationale for combining immunotoxins with cancer vaccines for the treatment of patients with advanced cancer.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures
Similar articles
-
Interleukin 13 mediates signal transduction through interleukin 13 receptor alpha2 in pancreatic ductal adenocarcinoma: role of IL-13 Pseudomonas exotoxin in pancreatic cancer therapy.Clin Cancer Res. 2010 Jan 15;16(2):577-86. doi: 10.1158/1078-0432.CCR-09-2015. Epub 2010 Jan 12. Clin Cancer Res. 2010. PMID: 20068108
-
Human adrenomedullin up-regulates interleukin-13 receptor alpha2 chain in prostate cancer in vitro and in vivo: a novel approach to sensitize prostate cancer to anticancer therapy.Cancer Res. 2008 Nov 15;68(22):9311-7. doi: 10.1158/0008-5472.CAN-08-2810. Cancer Res. 2008. PMID: 19010904 Free PMC article.
-
A Novel Recombinant Modified Vaccinia Ankara Virus expressing Interleukin-13 Receptor α2 Antigen for Potential Cancer Immunotherapy.Curr Mol Med. 2024;24(6):758-770. doi: 10.2174/1566524023666230331085007. Curr Mol Med. 2024. PMID: 36999709
-
IL-13 receptor-directed cancer vaccines and immunotherapy.Immunotherapy. 2012 Apr;4(4):443-51. doi: 10.2217/imt.12.28. Immunotherapy. 2012. PMID: 22512637 Review.
-
Recent advances with Treg depleting fusion protein toxins for cancer immunotherapy.Immunotherapy. 2019 Sep;11(13):1117-1128. doi: 10.2217/imt-2019-0060. Epub 2019 Jul 30. Immunotherapy. 2019. PMID: 31361167 Free PMC article. Review.
Cited by
-
IL-13Rα2 Regulates the IL-13/IFN-γ Balance during Innate Lymphoid Cell and Dendritic Cell Responses to Pox Viral Vector-Based Vaccination.Vaccines (Basel). 2021 May 1;9(5):440. doi: 10.3390/vaccines9050440. Vaccines (Basel). 2021. PMID: 34062727 Free PMC article.
-
Recent Advances in IL-13Rα2-Directed Cancer Immunotherapy.Front Immunol. 2022 Apr 8;13:878365. doi: 10.3389/fimmu.2022.878365. eCollection 2022. Front Immunol. 2022. PMID: 35464460 Free PMC article. Review.
-
Myeloid derived suppressor cells - a new therapeutic target in the treatment of cancer.J Immunother Cancer. 2013 Jul 15;1:10. doi: 10.1186/2051-1426-1-10. eCollection 2013. J Immunother Cancer. 2013. PMID: 24829747 Free PMC article. Review.
-
Fallen angels or risen apes? A tale of the intricate complexities of imbalanced immune responses in the pathogenesis and progression of immune-mediated and viral cancers.Front Immunol. 2014 Mar 6;5:90. doi: 10.3389/fimmu.2014.00090. eCollection 2014. Front Immunol. 2014. PMID: 24639678 Free PMC article. Review.
-
Ovarian cancer ascites proteomic profile reflects metabolic changes during disease progression.Biochem Biophys Rep. 2024 Jun 13;39:101755. doi: 10.1016/j.bbrep.2024.101755. eCollection 2024 Sep. Biochem Biophys Rep. 2024. PMID: 38974022 Free PMC article.
References
-
- Rice J, Ottensmeier CH, Stevenson FK. DNA vaccines: precision tools for activating effective immunity against cancer. Nat. Rev. Cancer. 2008;8:108–120. - PubMed
-
- Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin. Cancer Res. 2011;17:3520–3526. - PubMed
-
- Emens LA, Jaffee EM. Leveraging the activity of tumor vaccines with cytotoxic chemotherapy. Cancer Res. 2005;65:8059–8064. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

