A neutralizing human monoclonal antibody protects african green monkeys from hendra virus challenge
- PMID: 22013123
- PMCID: PMC3313625
- DOI: 10.1126/scitranslmed.3002901
A neutralizing human monoclonal antibody protects african green monkeys from hendra virus challenge
Abstract
Hendra virus (HeV) is a recently emerged zoonotic paramyxovirus that can cause a severe and often fatal disease in horses and humans. HeV is categorized as a biosafety level 4 agent, which has made the development of animal models and testing of potential therapeutics and vaccines challenging. Infection of African green monkeys (AGMs) with HeV was recently demonstrated, and disease mirrored fatal HeV infection in humans, manifesting as a multisystemic vasculitis with widespread virus replication in vascular tissues and severe pathologic manifestations in the lung, spleen, and brain. Here, we demonstrate that m102.4, a potent HeV-neutralizing human monoclonal antibody (hmAb), can protect AGMs from disease after infection with HeV. Fourteen AGMs were challenged intratracheally with a lethal dose of HeV, and 12 subjects were infused twice with a 100-mg dose of m102.4 beginning at either 10, 24, or 72 hours after infection and again about 48 hours later. The presence of viral RNA, infectious virus, and HeV-specific immune responses demonstrated that all subjects were infected after challenge. All 12 AGMs that received m102.4 survived infection, whereas the untreated control subjects succumbed to disease on day 8 after infection. Animals in the 72-hour treatment group exhibited neurological signs of disease, but all animals started to recover by day 16 after infection. These results represent successful post-exposure in vivo efficacy by an investigational drug against HeV and highlight the potential impact a hmAb can have on human disease.
Conflict of interest statement
Competing interests: C.C.B. and D.S.D. are United States federal employees, and D.S.D., Z.Z. and C.C.B. are coinventors on United States patent 7,988,971, Human monoclonal antibodies against Hendra and Nipah viruses; assignees are The United States of America as represented by the Department of Health and Human Services (Washington, DC), Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. (Bethesda, MD). All other authors declare no competing interests. The opinions or assertions contained herein are the private ones of the author(s) and are not to be construed as official or reflecting the views of the Department of Defense, the Uniformed Services University of Health Sciences, and the National Institutes of Allergy and Infectious Diseases, National Institutes of Health.
Figures
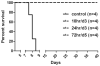
 ) in this group was sampled on day 27 and 35. No sample (*) was collected for one animal (
) in this group was sampled on day 27 and 35. No sample (*) was collected for one animal (
 ) on day 24.
) on day 24.References
-
- Mayo MA. A summary of taxonomic changes recently approved by ICTV. Arch Virol. 2002;147:1655. - PubMed
-
- Field H, Young P, Yob JM, Mills J, Hall L, Mackenzie J. The natural history of Hendra and Nipah viruses. Microbes Infect. 2001;3:307. - PubMed
-
- Bossart KN, Tachedjian M, McEachern JA, Crameri G, Zhu Z, Dimitrov DS, Broder CC, Wang LF. Functional studies of host-specific ephrin-B ligands as Henipavirus receptors. Virology. 2008;372:357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

