PRION-1 scales analysis supports use of functional outcome measures in prion disease
- PMID: 22013183
- PMCID: PMC3208955
- DOI: 10.1212/WNL.0b013e3182364890
PRION-1 scales analysis supports use of functional outcome measures in prion disease
Erratum in
- Neurology. 2012 Jan 31;78(5):371
Abstract
Objectives: Human prion diseases are heterogeneous but invariably fatal neurodegenerative disorders with no known effective therapy. PRION-1, the largest clinical trial in prion disease to date, showed no effect of the potential therapeutic quinacrine on survival. Although there are several limitations to the usefulness of survival as an outcome measure, there have been no comprehensive studies of alternatives.
Methods: To address this we did comparative analyses of neurocognitive, psychiatric, global, clinician-rated, and functional scales, focusing on validity, variability, and impact on statistical power over 77 person-years follow-up in 101 symptomatic patients in PRION-1.
Results: Quinacrine had no demonstrable benefit on any of the 8 scales (p > 0.4). All scales had substantial numbers of patients with the worst possible score at enrollment (Glasgow Coma Scale score being least affected) and were impacted by missing data due to disease progression. These effects were more significant for cognitive/psychiatric scales than global, clinician-rated, or functional scales. The Barthel and Clinical Dementia Rating scales were the most valid and powerful in simulated clinical trials of an effective therapeutic. A combination of selected subcomponents from these 2 scales gave somewhat increased power, compared to use of survival, to detect clinically relevant effects in future clinical trials of feasible size.
Conclusions: Our findings have implications for the choice of primary outcome measure in prion disease clinical trials. Prion disease presents the unusual opportunity to follow patients with a neurodegenerative disease through their entire clinical course, and this provides insights relevant to designing outcome measures in related conditions.
Figures
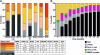
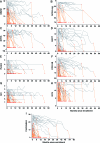

References
-
- Stewart LA, Rydzewska LH, Keogh GF, Knight RS. Systematic review of therapeutic interventions in human prion disease. Neurology 2008;70:1272–1281 - PubMed
-
- Collinge J. Prion diseases of humans and animals: their causes and molecular basis. Annu Rev Neurosci 2001;24:519–550 - PubMed
-
- Consumer workshop on clinical trials for CJD. Department of Health, Medical Research Council; 2002
-
- Bone I, Belton L, Walker AS, Darbyshire J. Intraventricular pentosan polysulphate in human prion disease: an observational study in the UK. Eur J Neurol 2008;15:458–464 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
