A new measure of cortical inhibition by mechanomyography and paired-pulse transcranial magnetic stimulation in unanesthetized rats
- PMID: 22013238
- PMCID: PMC3289479
- DOI: 10.1152/jn.00690.2011
A new measure of cortical inhibition by mechanomyography and paired-pulse transcranial magnetic stimulation in unanesthetized rats
Abstract
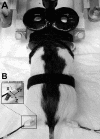
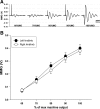
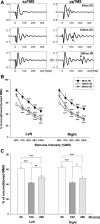
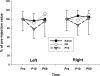
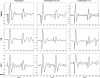
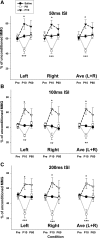
Paired-pulse transcranial magnetic stimulation (ppTMS) is a safe and noninvasive tool for measuring cortical inhibition in humans, particularly in patients with disorders of cortical inhibition such as epilepsy. However, ppTMS protocols in rodent disease models, where mechanistic insight into the ppTMS physiology and into disease processes may be obtained, have been limited due to the requirement for anesthesia and needle electromyography. To eliminate the confounding factor of anesthesia and to approximate human ppTMS protocols in awake rats, we adapted the mechanomyogram (MMG) method to investigate the ppTMS inhibitory phenomenon in awake rats and then applied differential pharmacology to test the hypothesis that long-interval cortical inhibition is mediated by the GABA(A) receptor. Bilateral hindlimb-evoked MMGs were elicited in awake rats by long-interval ppTMS protocols with 50-, 100-, and 200-ms interstimulus intervals. Acute changes in ppTMS-MMG were measured before and after intraperitoneal injections of saline, the GABA(A) agonist pentobarbital (PB), and GABA(A) antagonist pentylenetetrazole (PTZ). An evoked MMG was obtained in 100% of animals by single-pulse stimulation, and ppTMS resulted in predictable inhibition of the test-evoked MMG. With increasing TMS intensity, MMG amplitudes increased in proportion to machine output to produce reliable input-output curves. Simultaneous recordings of electromyography and MMG showed a predictable latency discrepancy between the motor-evoked potential and the evoked MMG (7.55 ± 0.08 and 9.16 ± 0.14 ms, respectively). With pharmacological testing, time course observations showed that ppTMS-MMG inhibition was acutely reduced following PTZ (P < 0.05), acutely enhanced after PB (P < 0.01) injection, and then recovered to pretreatment baseline after 1 h. Our data support the application of the ppTMS-MMG technique for measuring the cortical excitability in awake rats and provide the evidence that GABA(A) receptor contributes to long-interval paired-pulse cortical inhibition. Thus ppTMS-MMG appears a well-tolerated biomarker for measuring GABA(A)-mediated cortical inhibition in rats.
Figures

References
-
- Badawy RA, Macdonell RA, Berkovic SF, Newton MR, Jackson GD. Predicting seizure control: cortical excitability and antiepileptic medication. Ann Neurol 67: 64–73, 2010a - PubMed
-
- Badawy RA, Macdonell RA, Jackson GD, Berkovic SF. Can changes in cortical excitability distinguish progressive from juvenile myoclonic epilepsy? Epilepsia 51: 2084–2088, 2010b - PubMed
-
- Chen R. Interactions between inhibitory and excitatory circuits in the human motor cortex. Exp Brain Res 154: 1–10, 2004 - PubMed
-
- Chen R, Tam A, Butefisch C, Corwell B, Ziemann U, Rothwell JC, Cohen LG. Intracortical inhibition and facilitation in different representations of the human motor cortex. J Neurophysiol 80: 2870–2881, 1998 - PubMed
-
- Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol 2: 145–156, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

