Measurement of calcium dissociation rates from troponin C in rigor skeletal myofibrils
- PMID: 22013424
- PMCID: PMC3190119
- DOI: 10.3389/fphys.2011.00070
Measurement of calcium dissociation rates from troponin C in rigor skeletal myofibrils
Abstract
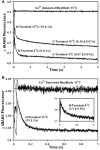
Ca2+ dissociation from the regulatory domain of troponin C may influence the rate of striated muscle relaxation. However, Ca(2+) dissociation from troponin C has not been measured within the geometric and stoichiometric constraints of the muscle fiber. Here we report the rates of Ca(2+) dissociation from the N-terminal regulatory and C-terminal structural domains of fluorescent troponin C constructs reconstituted into rabbit rigor psoas myofibrils using stopped-flow technology. Chicken skeletal troponin C fluorescently labeled at Cys 101, troponin C(IAEDANS), reported Ca(2+) dissociation exclusively from the structural domain of troponin C at ∼0.37, 0.06, and 0.07/s in isolation, in the presence of troponin I and in myofibrils at 15°C, respectively. Ca(2+) dissociation from the regulatory domain was observed utilizing fluorescently labeled troponin C containing the T54C and C101S mutations. Troponin [Formula: see text] reported Ca(2+) dissociation exclusively from the regulatory domain of troponin C at >1000, 8.8, and 15/s in isolation, in the presence of troponin I and in myofibrils at 15°C, respectively. Interestingly, troponin [Formula: see text] reported a biphasic fluorescence change upon Ca(2+) dissociation from the N- and C-terminal domains of troponin C with rates that were similar to those reported by troponin [Formula: see text] and troponin C(IAEDANS) at all levels of the troponin C systems. Furthermore, the rate of Ca(2+) dissociation from troponin C in the myofibrils was similar to the rate of Ca(2+) dissociation measured from the troponin C-troponin I complexes. Since the rate of Ca(2+) dissociation from the regulatory domain of TnC in myofibrils is similar to the rate of skeletal muscle relaxation, Ca(2+) dissociation from troponin C may influence relaxation.
Keywords: calcium; dissociation; muscle; myofibril; relaxation; skeletal; troponin.
Figures
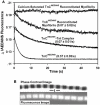
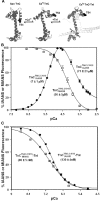

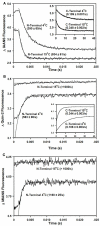
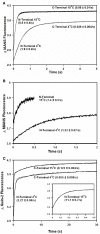
References
-
- Davis J. P., Rall J. A., Alionte C., Tikunova S. B. (2004). Mutations of hydrophobic residues in the N-terminal domain of troponin C affect calcium binding and exchange with the troponin C-troponin I96-148 complex and muscle force production. J. Biol. Chem. 279, 17348–1736010.1074/jbc.M410830200 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

