Triple-target treatment versus low-frequency electrostimulation for anal incontinence: a randomized, controlled trial
- PMID: 22013492
- PMCID: PMC3196995
- DOI: 10.3238/arztebl.2011.0653
Triple-target treatment versus low-frequency electrostimulation for anal incontinence: a randomized, controlled trial
Abstract
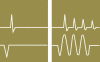
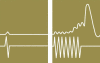
Background: In the nonsurgical treatment of anal incontinence, the combination of amplitude-modulated medium-frequency stimulation and electromyographic biofeedback (EMG-BF), known as triple-target treatment (3T), is superior to EMG-BF alone. The aim of this trial is to compare 3T with the standard treatment, low-frequency stimulation (LFS).
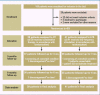
Methods: 80 patients with anal incontinence of Grade I or higher who presented to physicians or centers specialized in coloproctology were enrolled in this multicenter randomized trial with blinded observer. The trial had an open parallel-group design. Randomization was performed centrally by telephone. The primary endpoint was the Cleveland Clinic Score (CCS) after self-training at home with either 3T or LFS in two 20-minute sessions per day for 6 months. The secondary endpoints included the proportion of patients regaining continence, and the patients' quality of life (QoL). On completion of the trial as planned, the results were evaluated with an intention-to-treat analysis.
Study registration: DRKS00000138 (http://register.germanctr.de).
Results: 39 patients were randomized to 3T, and 41 to LFS. After 6 months of treatment, the CCS (mean ± standard deviation) was 3.1 ± 4.2 in the 3T group and 9.6 ± 3.9 in the LFS group. The median improvement in the CCS at 6 months compared to baseline was 7 points greater in the 3T group than in the LFS group (95% CI: 5-9, p<0.001). Anal continence was regained by 54% of the 3T patients, but none of the LFS patients (95% CI for the difference: 37.18% - 69.91%, p<0.001). QoL scores were higher in all dimensions in the 3T group than in the LFS group. No major adverse effects occurred in either group.
Conclusion: 3T is superior to LFS in the treatment of anal incontinence. The available evidence suggests that the success of 3T is based on the combined effect of biofeedback and medium-frequency stimulation. LFS of the type applied in this trial has no effect. 3T should be used in routine clinical practice instead of LFS.
Figures
Comment in
-
Alternative approaches to the treatment of fecal incontinence.Dtsch Arztebl Int. 2011 Sep;108(39):651-2. doi: 10.3238/arztebl.2011.0651. Epub 2011 Sep 30. Dtsch Arztebl Int. 2011. PMID: 22013491 Free PMC article. No abstract available.
References
-
- National Institute for Health and Clinical Excellence. Clinical Guideline CG 49. 2007. Faecal incontinence: The management of faecal incontinence in adults; pp. 309–310. web: www.nice.co.uk [04.06.2011]
-
- Spitzenverband Bund der Krankenkassen. web: www.gkv-spitzenverband.de. Hilfsmittelverzeichnis §139 SGB V; Neufassung Produktgruppe 09 „Elektrostimulation/-therapie vom 22. 11. 2007, Bundesanzeiger Nr. 218, Abschnitt PG 09.37.03; 2007. [Last accessed on 4 June 2011]
-
- Enck P, Van der Voort IR, Klosterhalfen S. Biofeedback therapy in fecal incontinence and constipation. Neurogastroenterol Motil. 2009;21:1133–1141. - PubMed
-
- Norton C, Cody JD, Hosker G. Biofeedback and/or sphincter exercises for the treatment of faecal incontinence in adults. Cochrane Database Syst Rev. 2006;3 CD002111. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

