Characterisation of the Trichinella spiralis deubiquitinating enzyme, TsUCH37, an evolutionarily conserved proteasome interaction partner
- PMID: 22013496
- PMCID: PMC3186758
- DOI: 10.1371/journal.pntd.0001340
Characterisation of the Trichinella spiralis deubiquitinating enzyme, TsUCH37, an evolutionarily conserved proteasome interaction partner
Abstract
Background: Trichinella spiralis is a zoonotic parasitic nematode that causes trichinellosis, a disease that has been identified on all continents except Antarctica. During chronic infection, T. spiralis larvae infect skeletal myofibres, severely disrupting their differentiation state.
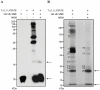
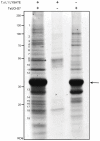
Methodology and results: An activity-based probe, HA-Ub-VME, was used to identify deubiquitinating enzyme (DUB) activity in lysate of T. spiralis L1 larvae. Results were analysed by immuno-blot and immuno-precipitation, identifying a number of potential DUBs. Immuno-precipitated proteins were subjected to LC/MS/MS, yielding peptides with sequence homology to 5 conserved human DUBs: UCH-L5, UCH-L3, HAUSP, OTU 6B and Ataxin-3. The predicted gene encoding the putative UCH-L5 homologue, TsUCH37, was cloned and recombinant protein was expressed and purified. The deubiquitinating activity of this enzyme was verified by Ub-AMC assay. Co-precipitation of recombinant TsUCH37 showed that the protein associates with putative T. spiralis proteasome components, including the yeast Rpn13 homologue ADRM1. In addition, the UCH inhibitor LDN-57444 exhibited specific inhibition of recombinant TsUCH37 and reduced the viability of cultured L1 larvae.
Conclusions: This study reports the identification of the first T. spiralis DUB, a cysteine protease that is putatively orthologous to the human protein, hUCH-L5. Results suggest that the interaction of this protein with the proteasome has been conserved throughout evolution. We show potential for the use of inhibitor compounds to elucidate the role of UCH enzymes in T. spiralis infection and their investigation as therapeutic targets for trichinellosis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Cloning, expression and characterisation of a cysteine protease from Trichinella spiralis.Folia Parasitol (Praha). 2018 May 29;65:2018.007. doi: 10.14411/fp.2018.007. Folia Parasitol (Praha). 2018. PMID: 29905572
-
Proteomic analysis of hydrolytic proteases in excretory/secretory proteins from Trichinella spiralis intestinal infective larvae using zymography combined with shotgun LC-MS/MS approach.Acta Trop. 2021 Apr;216:105825. doi: 10.1016/j.actatropica.2021.105825. Epub 2021 Jan 7. Acta Trop. 2021. PMID: 33421420
-
Molecular characterization of a cathepsin F-like protease in Trichinella spiralis.Parasit Vectors. 2015 Dec 21;8:652. doi: 10.1186/s13071-015-1270-y. Parasit Vectors. 2015. PMID: 26692208 Free PMC article.
-
Molecular identification and characterization of Trichinella spiralis proteasome subunit beta type-7.Parasit Vectors. 2015 Jan 13;8:18. doi: 10.1186/s13071-014-0626-z. Parasit Vectors. 2015. PMID: 25582125 Free PMC article.
-
Trichinella spiralis: genomic application to control a zoonotic nematode.Infect Disord Drug Targets. 2010 Oct;10(5):376-84. doi: 10.2174/187152610793180830. Infect Disord Drug Targets. 2010. PMID: 20701572 Review.
Cited by
-
Molecular Docking and In Silico Simulation of Trichinella spiralis Membrane-Associated Progesterone Receptor Component 2 (Ts-MAPRC2) and Its Interaction with Human PGRMC1.Biomed Res Int. 2022 Jun 20;2022:7414198. doi: 10.1155/2022/7414198. eCollection 2022. Biomed Res Int. 2022. PMID: 35769668 Free PMC article.
-
Ubiquitin recognition of BAP1: understanding its enzymatic function.Biosci Rep. 2017 Oct 27;37(5):BSR20171099. doi: 10.1042/BSR20171099. Print 2017 Oct 31. Biosci Rep. 2017. PMID: 28935764 Free PMC article.
-
Nedd8 hydrolysis by UCH proteases in Plasmodium parasites.PLoS Pathog. 2019 Oct 28;15(10):e1008086. doi: 10.1371/journal.ppat.1008086. eCollection 2019 Oct. PLoS Pathog. 2019. PMID: 31658303 Free PMC article.
-
Ubiquitin-Dependent Modification of Skeletal Muscle by the Parasitic Nematode, Trichinella spiralis.PLoS Pathog. 2016 Nov 21;12(11):e1005977. doi: 10.1371/journal.ppat.1005977. eCollection 2016 Nov. PLoS Pathog. 2016. PMID: 27870901 Free PMC article.
-
Detection of ubiquitin-proteasome enzymatic activities in cells: application of activity-based probes to inhibitor development.Biochim Biophys Acta. 2012 Nov;1823(11):2029-37. doi: 10.1016/j.bbamcr.2012.05.014. Epub 2012 May 19. Biochim Biophys Acta. 2012. PMID: 22613766 Free PMC article. Review.
References
-
- Dupouy Camet JM, KD Dupouy-Camet J, Murrell KD, editors. FAO/WHO/OIE guidelines for the surveillance, management, prevention and control of trichinellosis. 2007. © Copyright FAO/WHO/OIE, 2007.
-
- Pozio E. New patterns of Trichinella infection. Vet Parasitol. 2001;98:133–148. - PubMed
-
- Wakelin D. Trichinella spiralis: immunity, ecology, and evolution. J Parasitol. 1993;79:488–494. - PubMed
-
- Compton SJ, Celum CL, Lee C, Thompson D, Sumi SM, et al. Trichinosis with ventilatory failure and persistent myocarditis. Clin Infect Dis. 1993;16:500–504. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

