Cytokine-induced alterations of gastrointestinal motility in gastrointestinal disorders
- PMID: 22013552
- PMCID: PMC3196622
- DOI: 10.4291/wjgp.v2.i5.72
Cytokine-induced alterations of gastrointestinal motility in gastrointestinal disorders
Abstract
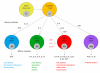
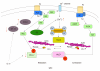
Inflammation and immune activation in the gut are usually accompanied by alteration of gastrointestinal (GI) motility. In infection, changes in motor function have been linked to host defense by enhancing the expulsion of the infectious agents. In this review, we describe the evidence for inflammation and immune activation in GI infection, inflammatory bowel disease, ileus, achalasia, eosinophilic esophagitis, microscopic colitis, celiac disease, pseudo-obstruction and functional GI disorders. We also describe the possible mechanisms by which inflammation and immune activation in the gut affect GI motility. GI motility disorder is a broad spectrum disturbance of GI physiology. Although several systems including central nerves, enteric nerves, interstitial cells of Cajal and smooth muscles contribute to a coordinated regulation of GI motility, smooth muscle probably plays the most important role. Thus, we focus on the relationship between activation of cytokines induced by adaptive immune response and alteration of GI smooth muscle contractility. Accumulated evidence has shown that Th1 and Th2 cytokines cause hypocontractility and hypercontractility of inflamed intestinal smooth muscle. Th1 cytokines downregulate CPI-17 and L-type Ca(2+) channels and upregulate regulators of G protein signaling 4, which contributes to hypocontractility of inflamed intestinal smooth muscle. Conversely, Th2 cytokines cause hypercontractilty via signal transducer and activator of transcription 6 or mitogen-activated protein kinase signaling pathways. Th1 and Th2 cytokines have opposing effects on intestinal smooth muscle contraction via 5-hydroxytryptamine signaling. Understanding the immunological basis of altered GI motor function could lead to new therapeutic strategies for GI functional and inflammatory disorders.
Keywords: Cytokine; Immunology; Inflammation; Motility.
Figures
References
-
- Bercík P, De Giorgio R, Blennerhassett P, Verdú EF, Barbara G, Collins SM. Immune-mediated neural dysfunction in a murine model of chronic Helicobacter pylori infection. Gastroenterology. 2002;123:1205–1215. - PubMed
-
- Vallance BA, Croitoru K, Collins SM. T lymphocyte-dependent and -independent intestinal smooth muscle dysfunction in the T. spiralis-infected mouse. Am J Physiol. 1998;275:G1157–G1165. - PubMed
-
- Myers BS, Dempsey DT, Yasar S, Martin JS, Parkman HP, Ryan JP. Acute experimental distal colitis alters colonic transit in rats. J Surg Res. 1997;69:107–112. - PubMed
-
- Krauter EM, Strong DS, Brooks EM, Linden DR, Sharkey KA, Mawe GM. Changes in colonic motility and the electrophysiological properties of myenteric neurons persist following recovery from trinitrobenzene sulfonic acid colitis in the guinea pig. Neurogastroenterol Motil. 2007;19:990–1000. - PubMed
-
- Radojevic N, McKay DM, Merger M, Vallance BA, Collins SM, Croitoru K. Characterization of enteric functional changes evoked by in vivo anti-CD3 T cell activation. Am J Physiol. 1999;276:R715–R723. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

