Comparative void-volume analysis of psychrophilic and mesophilic enzymes: Structural bioinformatics of psychrophilic enzymes reveals sources of core flexibility
- PMID: 22013889
- PMCID: PMC3224250
- DOI: 10.1186/1472-6807-11-42
Comparative void-volume analysis of psychrophilic and mesophilic enzymes: Structural bioinformatics of psychrophilic enzymes reveals sources of core flexibility
Abstract
Background: Psychrophiles, cold-adapted organisms, have adapted to live at low temperatures by using a variety of mechanisms. Their enzymes are active at cold temperatures by being structurally more flexible than mesophilic enzymes. Even though, there are some indications of the possible structural mechanisms by which psychrophilic enzymes are catalytic active at cold temperatures, there is not a generalized structural property common to all psychrophilic enzymes.
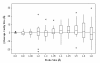
Results: We examine twenty homologous enzyme pairs from psychrophiles and mesophiles to investigate flexibility as a key characteristic for cold adaptation. B-factors in protein X-ray structures are one way to measure flexibility. Comparing psychrophilic to mesophilic protein B-factors reveals that psychrophilic enzymes are more flexible in 5-turn and strand secondary structures. Enzyme cavities, identified using CASTp at various probe sizes, indicate that psychrophilic enzymes have larger average cavity sizes at probe radii of 1.4-1.5 Å, sufficient for water molecules. Furthermore, amino acid side chains lining these cavities show an increased frequency of acidic groups in psychrophilic enzymes.
Conclusions: These findings suggest that embedded water molecules may play a significant role in cavity flexibility, and therefore, overall protein flexibility. Thus, our results point to the important role enzyme flexibility plays in adaptation to cold environments.
Figures
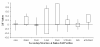

References
-
- Vogt G, Argos P. Protein thermal stability: hydrogen bonds or internal packing. J Mol Biol. 1997;269:631–643. - PubMed
-
- Chen J, Stites WE. Replacement of staphylococcal nuclease hydrophobic core residues with those from thermophilic homologues indicates packing is improved in some thermostable proteins. J Mol Biol. 2004;344:271–280. - PubMed
-
- Cavicchioli R, Siddiqui KS, Andrews D, Sowers KR. Low-temperature extremophiles and their applications. Current Opinion in Biotechnology. 2002;13:253–261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

