Novel cAMP signalling paradigms: therapeutic implications for airway disease
- PMID: 22013890
- PMCID: PMC3417475
- DOI: 10.1111/j.1476-5381.2011.01719.x
Novel cAMP signalling paradigms: therapeutic implications for airway disease
Abstract
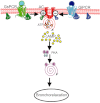
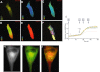
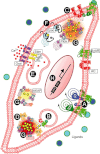
Since its discovery over 50 years ago, cAMP has been the archetypal second messenger introducing students to the concept of cell signalling at the simplest level. As explored in this review, however, there are many more facets to cAMP signalling than the path from Gs-coupled receptor to adenylyl cyclase (AC) to cAMP to PKA to biological effect. After a brief description of this canonical cAMP signalling pathway, a snapshot is provided of the novel paradigms of cAMP signalling. As in the airway the cAMP pathway relays the major bronchorelaxant signal and as such is the target for frontline therapy for asthma and COPD, particular emphasis is given to airway disease and therapy. Areas discussed include biased agonism, continued signalling following internalization, modulation of cAMP by AC, control of cAMP degradation, cAMP and calcium crosstalk, Epac-mediated signalling and finally the implications of altered genotypes will be considered. LINKED ARTICLES This article is part of a themed section on Novel cAMP Signalling Paradigms. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2012.166.issue-2.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures
Similar articles
-
cAMP and Epac in the regulation of tissue fibrosis.Br J Pharmacol. 2012 May;166(2):447-56. doi: 10.1111/j.1476-5381.2012.01847.x. Br J Pharmacol. 2012. PMID: 22233238 Free PMC article. Review.
-
Hydrogen peroxide stimulation of CFTR reveals an Epac-mediated, soluble AC-dependent cAMP amplification pathway common to GPCR signalling.Br J Pharmacol. 2015 Jan;172(1):173-84. doi: 10.1111/bph.12934. Epub 2014 Dec 15. Br J Pharmacol. 2015. PMID: 25220136 Free PMC article.
-
Cyclic Adenosine 3',5'-Monophosphate Elevation and Biological Signaling through a Secretin Family Gs-Coupled G Protein-Coupled Receptor Are Restricted to a Single Adenylate Cyclase Isoform.Mol Pharmacol. 2015 Jun;87(6):928-35. doi: 10.1124/mol.115.098087. Epub 2015 Mar 13. Mol Pharmacol. 2015. PMID: 25769305 Free PMC article.
-
Soluble adenylyl cyclase-mediated cAMP signaling and the putative role of PKA and EPAC in cerebral mitochondrial function.J Neurosci Res. 2019 Aug;97(8):1018-1038. doi: 10.1002/jnr.24477. Epub 2019 Jun 6. J Neurosci Res. 2019. PMID: 31172581
-
Distinct PKA and Epac compartmentalization in airway function and plasticity.Pharmacol Ther. 2013 Feb;137(2):248-65. doi: 10.1016/j.pharmthera.2012.10.006. Epub 2012 Oct 23. Pharmacol Ther. 2013. PMID: 23089371 Review.
Cited by
-
Searching for synergistic bronchodilators and novel therapeutic regimens for chronic lung diseases from a traditional Chinese medicine, Qingfei Xiaoyan Wan.PLoS One. 2014 Nov 14;9(11):e113104. doi: 10.1371/journal.pone.0113104. eCollection 2014. PLoS One. 2014. PMID: 25397687 Free PMC article.
-
cAMP regulation of airway smooth muscle function.Pulm Pharmacol Ther. 2013 Feb;26(1):112-20. doi: 10.1016/j.pupt.2012.05.007. Epub 2012 May 24. Pulm Pharmacol Ther. 2013. PMID: 22634112 Free PMC article. Review.
-
No evidence for altered intracellular calcium-handling in airway smooth muscle cells from human subjects with asthma.BMC Pulm Med. 2015 Feb 13;15:12. doi: 10.1186/s12890-015-0009-z. BMC Pulm Med. 2015. PMID: 25880173 Free PMC article.
-
The Concise Guide to PHARMACOLOGY 2013/14: enzymes.Br J Pharmacol. 2013 Dec;170(8):1797-867. doi: 10.1111/bph.12451. Br J Pharmacol. 2013. PMID: 24528243 Free PMC article.
-
Signaling Pathways Used by the Specialized Pro-Resolving Mediator Maresin 2 Regulate Goblet Cell Function: Comparison with Maresin 1.Int J Mol Sci. 2022 Jun 2;23(11):6233. doi: 10.3390/ijms23116233. Int J Mol Sci. 2022. PMID: 35682912 Free PMC article.
References
-
- Ay B, Iyanoye A, Sieck GC, Prakash YS, Pabelick CM. Cyclic nucleotide regulation of store-operated Ca2+ influx in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2006;290:L278–L283. - PubMed
-
- Beavo JA, Brunton LL. Cyclic nucleotide research – still expanding after half a century. Nat Rev Mol Cell Biol. 2002;3:710–718. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

