Blockade of I(Ca) suppresses early afterdepolarizations and reduces transmural dispersion of repolarization in a whole heart model of chronic heart failure
- PMID: 22013922
- PMCID: PMC3417488
- DOI: 10.1111/j.1476-5381.2011.01721.x
Blockade of I(Ca) suppresses early afterdepolarizations and reduces transmural dispersion of repolarization in a whole heart model of chronic heart failure
Abstract
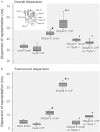
BACKGROUND AND PURPOSE Chronic heart failure (CHF) is associated with action potential prolongation and Ca(2+) overload, increasing risk of ventricular tachyarrhythmias (VT). We therefore investigated whether I(Ca) blockade was anti-arrhythmic in an intact perfused heart model of CHF. EXPERIMENTAL APPROACH CHF was induced in rabbits after 4 weeks of rapid ventricular pacing. Hearts from CHF and sham-operated rabbits were isolated and perfused (Langendorff preparation), with ablation of the AV node. VT was induced by erythromycin and low [K(+) ] (1.5mM). Electrophysiology of cardiac myocytes, with block of cation currents, was simulated by a mathematical model. KEY RESULTS Repolarization was prolonged in CHF hearts compared with sham-operated hearts. Action potential duration (APD) and overall dispersion of repolarization were further increased by erythromycin (300 µM) to block I(Kr) in CHF hearts. After lowering [K(+) ] to 1.5mM, CHF and sham hearts showed spontaneous episodes of polymorphic non-sustained VT. Additional infusion of verapamil (0.75 µM) suppressed early afterdepolarizations (EAD) and VT in 75% of sham and CHF hearts. Verapamil shortened APD and dispersion of repolarization, mainly by reducing transmural dispersion of repolarization via shortening of endocardial action potentials. Mathematical simulations showed that EADs were more effectively reduced by verapamil assuming a state-dependent block than a simple block of I(Ca) . CONCLUSIONS AND IMPLICATIONS Blockade of I(Ca) was highly effective in suppressing VT via reduction of transmural dispersion of repolarization and suppression of EAD. Such blockade might represent a novel therapeutic option to reduce risk of VT in structurally normal hearts and also in heart failure. LINKED ARTICLE This article is commented on by Stams et al., pp. 554-556 of this issue. To view this commentary visit http://dx.doi.org/10.1111/j.1476-5381.2011.01818.x.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures





References
-
- Akar FG, Rosenbaum DS. Transmural electrophysiological heterogeneities underlying arrhythmogenesis in heart failure. Circ Res. 2003;93:638–645. - PubMed
-
- Antzelevitch C, Sun ZQ, Zhang ZQ, Yan GX. Cellular and ionic mechanisms underlying erythromycin-induced long QT intervals and torsade de pointes. J Am Coll Cardiol. 1996;28:1836–1848. - PubMed
-
- Barr CS, Naas A, Freeman M, Lang CC, Struthers AD. QT dispersion and sudden unexpected death in chronic heart failure. Lancet. 1994;343:327–329. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

