Cyclic nucleotide phosphodiesterase (PDE) isozymes as targets of the intracellular signalling network: benefits of PDE inhibitors in various diseases and perspectives for future therapeutic developments
- PMID: 22014080
- PMCID: PMC3372715
- DOI: 10.1111/j.1476-5381.2011.01729.x
Cyclic nucleotide phosphodiesterase (PDE) isozymes as targets of the intracellular signalling network: benefits of PDE inhibitors in various diseases and perspectives for future therapeutic developments
Abstract
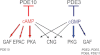
Cyclic nucleotide phosphodiesterases (PDEs) that specifically inactivate the intracellular messengers cAMP and cGMP in a compartmentalized manner represent an important enzyme class constituted by 11 gene-related families of isozymes (PDE1 to PDE11). Downstream receptors, PDEs play a major role in controlling the signalosome at various levels of phosphorylations and protein/protein interactions. Due to the multiplicity of isozymes, their various intracellular regulations and their different cellular and subcellular distributions, PDEs represent interesting targets in intracellular pathways. Therefore, the investigation of PDE isozyme alterations related to various pathologies and the design of specific PDE inhibitors might lead to the development of new specific therapeutic strategies in numerous pathologies. This manuscript (i) overviews the different PDEs including their endogenous regulations and their specific inhibitors; (ii) analyses the intracellular implications of PDEs in regulating signalling cascades in pathogenesis, exemplified by two diseases affecting cell cycle and proliferation; and (iii) discusses perspectives for future therapeutic developments.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures
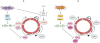

References
-
- Abusnina A, Alhosin M, Keravis T, Muller CD, Fuhrmann G, Bronner C, et al. Down-regulation of cyclic nucleotide phosphodiesterase PDE1A is the key event of p73 and UHRF1 deregulation in thymoquinone-induced acute lymphoblastic leukemia cell apoptosis. Cell Signal. 2011;23:152–160. - PubMed
-
- Ahmad F, Lindh R, Tang Y, Ruishalme I, Ost A, Sahachartsiri B, et al. Differential regulation of adipocyte PDE3B in distinct membrane compartments by insulin and the beta3-adrenergic receptor agonist CL316243: effects of caveolin-1 knockdown on formation/maintenance of macromolecular signalling complexes. Biochem J. 2009;424:399–410. - PMC - PubMed
-
- Ahn HS, Foster M, Foster C, Sybertz E, Wells JN. Effects of selective inhibitors on cyclic nucleotide phosphodiesterases of rabbit aorta. Biochem Pharmacol. 1989;38:3331–3339. - PubMed
-
- Ahn HS, Bercovici A, Boykow G, Bronnenkant A, Chackalamannil S, Chow J, et al. Potent tetracyclic guanine inhibitors of PDE1 and PDE5 cyclic guanosine monophosphate phosphodiesterases with oral antihypertensive activity. J Med Chem. 1997;40:2196–2210. - PubMed
-
- Alhosin M, Abusnina A, Achour M, Sharif T, Muller C, Peluso J, et al. Induction of apoptosis by thymoquinone in lymphoblastic leukemia Jurkat cells is mediated by a p73-dependent pathway which targets the epigenetic integrator UHRF1. Biochem Pharmacol. 2010;79:1251–1260. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

