Effects of magnolol on UVB-induced skin cancer development in mice and its possible mechanism of action
- PMID: 22014088
- PMCID: PMC3234292
- DOI: 10.1186/1471-2407-11-456
Effects of magnolol on UVB-induced skin cancer development in mice and its possible mechanism of action
Abstract
Background: Magnolol, a plant lignan isolated from the bark and seed cones of Magnolia officinalis, has been shown to have chemopreventive effects on chemically-induced skin cancer development. The objectives of this investigation are to study the anticarcinogenic effects of magnolol on UVB-induced skin tumor development in SKH-1 mice, a model relevant to humans, and determine the possible role of apoptosis and cell cycle arrest involved in the skin tumor development.
Methods: UVB-induced skin carcinogenesis model in SKH-1 mice was used for determining the preventive effects of magnolol on skin cancer development. Western blottings and flow cytometric analysis were used to study the effects of magnolol on apoptosis and cell cycle.
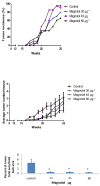
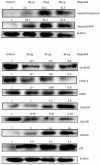
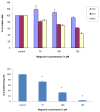
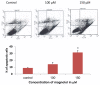
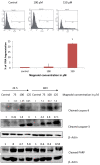
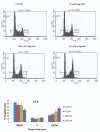
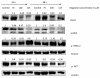
Results: Magnolol pretreated groups (30, 60 μ g) before UVB treatments (30 mJ/cm2, 5 days/week) resulted in 27-55% reduction in tumor multiplicity as compared to control group in SKH-1 mice. Magnolol pretreatment increased the cleavage of caspase-8 and poly-(-ADP-ribose) polymerase (PARP), increased the expression of p21, a cell cycle inhibitor, and decreased the expression of proteins involved in the G2/M phase of cell cycle in skin samples from SKH-1 mice.Treatment of A431 cells with magnolol decreased cell viability and cell proliferation in a concentration dependent manner. Magnolol induced G2/M phase cell cycle arrest in A431 cells at 12 h with a decreased expression of cell cycle proteins such as cyclin B1, cyclin A, CDK4, Cdc2 and simultaneous increase in the expression of Cip/p21, a cyclin-dependent kinase inhibitor. Magnolol induced apoptosis in vivo and in vitro with an increased cleavage of caspase-8 and PARP. Phospho-signal transducers and activators of transcription 3 (Tyr705), B-Raf, p-MEK, and p-AKT were down-regulated, whereas phosphorylation of ERK was induced by magnolol in A431 cells.
Conclusions: Magnolol pretreatments prevent UVB-induced skin cancer development by enhancing apoptosis, causing cell cycle arrest at G2/M phase, and affecting various signaling pathways. Magnolol could be a potentially safe and potent anticarcinogenic agent against skin cancer.
Figures



Similar articles
-
Honokiol, a chemopreventive agent against skin cancer, induces cell cycle arrest and apoptosis in human epidermoid A431 cells.Exp Biol Med (Maywood). 2011 Nov;236(11):1351-9. doi: 10.1258/ebm.2011.011030. Epub 2011 Sep 9. Exp Biol Med (Maywood). 2011. PMID: 21908486
-
Chemopreventive effects of honokiol on UVB-induced skin cancer development.Anticancer Res. 2010 Mar;30(3):777-83. Anticancer Res. 2010. PMID: 20392996
-
Chemopreventive effects of combination of honokiol and magnolol with α-santalol on skin cancer developments.Drug Discov Ther. 2013 Jun;7(3):109-15. Drug Discov Ther. 2013. PMID: 23917859
-
Magnolol as a Potential Anticancer Agent: A Proposed Mechanistic Insight.Molecules. 2022 Sep 29;27(19):6441. doi: 10.3390/molecules27196441. Molecules. 2022. PMID: 36234977 Free PMC article. Review.
-
Skin cancer chemoprevention by α-santalol.Front Biosci (Schol Ed). 2011 Jan 1;3(2):777-87. doi: 10.2741/s186. Front Biosci (Schol Ed). 2011. PMID: 21196411 Review.
Cited by
-
Magnolol: A Neolignan from the Magnolia Family for the Prevention and Treatment of Cancer.Int J Mol Sci. 2018 Aug 10;19(8):2362. doi: 10.3390/ijms19082362. Int J Mol Sci. 2018. PMID: 30103472 Free PMC article. Review.
-
Role of magnolol in the proliferation of vascular smooth muscle cells.Herz. 2015 May;40(3):542-8. doi: 10.1007/s00059-014-4051-z. Epub 2014 Mar 6. Herz. 2015. PMID: 24595318
-
Magnolol inhibits growth of gallbladder cancer cells through the p53 pathway.Cancer Sci. 2015 Oct;106(10):1341-50. doi: 10.1111/cas.12762. Cancer Sci. 2015. PMID: 26250568 Free PMC article.
-
2-O-Methylmagnolol upregulates the long non-coding RNA, GAS5, and enhances apoptosis in skin cancer cells.Cell Death Dis. 2017 Mar 2;8(3):e2638. doi: 10.1038/cddis.2017.66. Cell Death Dis. 2017. PMID: 28252643 Free PMC article.
-
Cisplatin-Induced Muscle Wasting and Atrophy: Molecular Mechanism and Potential Therapeutic Interventions.J Cachexia Sarcopenia Muscle. 2025 Jun;16(3):e13817. doi: 10.1002/jcsm.13817. J Cachexia Sarcopenia Muscle. 2025. PMID: 40343378 Free PMC article. Review.
References
-
- Lu YP, Lou YR, Yen P, Mitchell D, Huang MT, Conney AH. Time course for early adaptive responses to ultraviolet B light in the epidermis of SKH-1 mice. Cancer Res. 1999. pp. 4591–4602. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

