Analysis of induced electrical currents from magnetic field coupling inside implantable neurostimulator leads
- PMID: 22014169
- PMCID: PMC3213036
- DOI: 10.1186/1475-925X-10-94
Analysis of induced electrical currents from magnetic field coupling inside implantable neurostimulator leads
Abstract
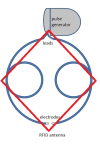
Background: Over the last decade, the number of neurostimulator systems implanted in patients has been rapidly growing. Nearly 50, 000 neurostimulators are implanted worldwide annually. The most common type of implantable neurostimulators is indicated for pain relief. At the same time, commercial use of other electromagnetic technologies is expanding, making electromagnetic interference (EMI) of neurostimulator function an issue of concern. Typically reported sources of neurostimulator EMI include security systems, metal detectors and wireless equipment. When near such sources, patients with implanted neurostimulators have reported adverse events such as shock, pain, and increased stimulation. In recent in vitro studies, radio frequency identification (RFID) technology has been shown to inhibit the stimulation pulse of an implantable neurostimulator system during low frequency exposure at close distances. This could potentially be due to induced electrical currents inside the implantable neurostimulator leads that are caused by magnetic field coupling from the low frequency identification system.
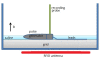
Methods: To systematically address the concerns posed by EMI, we developed a test platform to assess the interference from coupled magnetic fields on implantable neurostimulator systems. To measure interference, we recorded the output of one implantable neurostimulator, programmed for best therapy threshold settings, when in close proximity to an operating low frequency RFID emitter. The output contained electrical potentials from the neurostimulator system and those induced by EMI from the RFID emitter. We also recorded the output of the same neurostimulator system programmed for best therapy threshold settings without RFID interference. Using the Spatially Extended Nonlinear Node (SENN) model, we compared threshold factors of spinal cord fiber excitation for both recorded outputs.
Results: The electric current induced by low frequency RFID emitter was not significant to have a noticeable effect on electrical stimulation.
Conclusions: We demonstrated a method for analyzing effects of coupled magnetic field interference on implantable neurostimulator system and its electrodes which could be used by device manufacturers during the design and testing phases of the development process.
Figures



References
-
- Finkenzeller K. RFID handbook: fundamentals and applications in contactless smart cards and identification. Chichester, West Sussex, England, John Wiley & Sons Ltd; 2003.
-
- U.S. Food and Drug Administration: Radiofrequency Identification (RFID) http://www.fda.gov/Radiation-EmittingProducts/RadiationSafety/Electromag...
-
- U.S. Food and Drug Administration: Radiofrequency Identification Technology: Protecting the Drug Supply. http://www.fda.gov/Drugs/DrugSafety/ucm169918.htm
-
- Christe B, Rogers R, Cooney E. Analysis of the Impact of a Radiofrequency Identification Asset-Tracking System in the Healthcare Setting. Journal of Clinical Engineering. 2010;35(1):49–55.
MeSH terms
LinkOut - more resources
Full Text Sources

