Correction of hypophosphatasia-associated mineralization deficiencies in vitro by phosphate/pyrophosphate modulation in periodontal ligament cells
- PMID: 22014174
- PMCID: PMC3871996
- DOI: 10.1902/jop.2011.110310
Correction of hypophosphatasia-associated mineralization deficiencies in vitro by phosphate/pyrophosphate modulation in periodontal ligament cells
Abstract
Background: Mutations in the liver/bone/kidney alkaline phosphatase (ALPL) gene in hypophosphatasia (HPP) reduce the function of tissue non-specific alkaline phosphatase (ALP), resulting in increased pyrophosphate (PP(i)) and a severe deficiency in acellular cementum. We hypothesize that exogenous phosphate (P(i)) would rescue the in vitro mineralization capacity of periodontal ligament (PDL) cells harvested from HPP-diagnosed patients, by correcting the P(i)/PP(i) ratio and modulating expression of genes involved with P(i)/PP(i) metabolism.
Methods: Ex vivo and in vitro analyses were used to identify mechanisms involved in HPP-associated PDL/tooth root deficiencies. Constitutive expression of PP(i)-associated genes was contrasted in PDL versus pulp tissues obtained from healthy individuals. Primary PDL cell cultures from patients with HPP (monozygotic twin males) were established to assay ALP activity, in vitro mineralization, and gene expression. Exogenous P(i) was provided to correct the P(i)/PP(i) ratio.
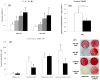
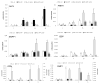
Results: PDL tissues obtained from healthy individuals featured higher basal expression of key PP(i) regulators, genes ALPL, progressive ankylosis protein (ANKH), and ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1), versus paired pulp tissues. A novel ALPL mutation was identified in the twin patients with HPP enrolled in this study. Compared to controls, HPP-PDL cells exhibited significantly reduced ALP and mineralizing capacity, which were rescued by addition of 1 mM P(i). Dysregulated expression of PP(i) regulatory genes ALPL, ANKH, and ENPP1 was also corrected by adding P(i), although other matrix markers evaluated in our study remained downregulated.
Conclusion: These findings underscore the importance of controlling the P(i)/PP(i) ratio toward development of a functional periodontal apparatus and support P(i)/PP(i) imbalance as the etiology of HPP-associated cementum defects.
Conflict of interest statement
Figures

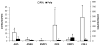
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

