Transient receptor potential melastatin 4 inhibitor 9-phenanthrol abolishes arrhythmias induced by hypoxia and re-oxygenation in mouse ventricle
- PMID: 22014185
- PMCID: PMC3413868
- DOI: 10.1111/j.1476-5381.2011.01715.x
Transient receptor potential melastatin 4 inhibitor 9-phenanthrol abolishes arrhythmias induced by hypoxia and re-oxygenation in mouse ventricle
Abstract
Background and purpose: Hypoxia and subsequent re-oxygenation are associated with cardiac arrhythmias such as early afterdepolarizations (EADs), which may be partly explained by perturbations in cytosolic calcium concentration. Transient receptor potential melastatin 4 (TRPM4), a calcium-activated non-selective cation channel, is functionally expressed in the heart. Based on its biophysical properties, it is likely to participate in EADs. Hence, modulators of TRPM4 activity may influence arrhythmias. The aim of this study was to investigate the possible anti-arrhythmic effect of 9-phenanthrol, a TRPM4 inhibitor in a murine heart model of hypoxia and re-oxygenation-induced EADs.
Experimental approach: Mouse heart was removed, and the right ventricle was pinned in a superfusion chamber. After a period of normoxia, the preparation was superfused for 2 h with a hypoxic solution and then re-oxygenated. Spontaneous electrical activity was investigated by intracellular microelectrode recordings.
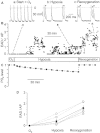
Key results: In normoxic conditions, the ventricle exhibited spontaneous action potentials. Application of the hypoxia and re-oxygenation protocol unmasked hypoxia-induced EADs, the occurrence of which increased under re-oxygenation. The frequency of these EADs was reduced by superfusion with either flufenamic acid, a blocker of Ca(2+) -dependent cation channels or with 9-phenanthrol. Superfusion with 9-phenanthrol (10(-5) or 10(-4) mol·L(-1) ) caused a dramatic dose-dependent abolition of EADs.
Conclusions and implications: Hypoxia and re-oxygenation-induced EADs can be generated in the mouse heart model. 9-Phenanthrol abolished EADs, which strongly suggests the involvement of TRPM4 in the generation of EAD. This identifies non-selective cation channels inhibitors as new pharmacological candidates in the treatment of arrhythmias.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures




Similar articles
-
Implication of the TRPM4 nonselective cation channel in mammalian sinus rhythm.Heart Rhythm. 2013 Nov;10(11):1683-9. doi: 10.1016/j.hrthm.2013.08.014. Epub 2013 Aug 14. Heart Rhythm. 2013. PMID: 23954346
-
Transient receptor potential melastatin 4 channel inhibitor 9-phenanthrol inhibits K+ but not Ca2+ currents in canine ventricular myocytes.Can J Physiol Pharmacol. 2018 Oct;96(10):1022-1029. doi: 10.1139/cjpp-2018-0049. Epub 2018 May 28. Can J Physiol Pharmacol. 2018. PMID: 29806985
-
9-Phenanthrol and flufenamic acid inhibit calcium oscillations in HL-1 mouse cardiomyocytes.Cell Calcium. 2013 Sep;54(3):193-201. doi: 10.1016/j.ceca.2013.06.003. Epub 2013 Jul 5. Cell Calcium. 2013. PMID: 23831210 Free PMC article.
-
The TRPM4 channel inhibitor 9-phenanthrol.Br J Pharmacol. 2014 Apr;171(7):1600-13. doi: 10.1111/bph.12582. Br J Pharmacol. 2014. PMID: 24433510 Free PMC article. Review.
-
TRPM4 in cardiac electrical activity.Cardiovasc Res. 2015 Oct 1;108(1):21-30. doi: 10.1093/cvr/cvv213. Epub 2015 Aug 13. Cardiovasc Res. 2015. PMID: 26272755 Review.
Cited by
-
Differential effects of TRPM4 channel inhibitors on Guinea pig urinary bladder smooth muscle excitability and contractility: Novel 4-chloro-2-[2-(2-chloro-phenoxy)-acetylamino]-benzoic acid (CBA) versus classical 9-phenanthrol.Pharmacol Res Perspect. 2022 Aug;10(4):e00982. doi: 10.1002/prp2.982. Pharmacol Res Perspect. 2022. PMID: 35822549 Free PMC article.
-
TRPM4 non-selective cation channels influence action potentials in rabbit Purkinje fibres.J Physiol. 2016 Jan 15;594(2):295-306. doi: 10.1113/JP271347. Epub 2015 Dec 14. J Physiol. 2016. PMID: 26548780 Free PMC article.
-
Pharmacological Modulation and (Patho)Physiological Roles of TRPM4 Channel-Part 1: Modulation of TRPM4.Pharmaceuticals (Basel). 2022 Jan 10;15(1):81. doi: 10.3390/ph15010081. Pharmaceuticals (Basel). 2022. PMID: 35056138 Free PMC article. Review.
-
Mechanobiology in cardiac physiology and diseases.J Cell Mol Med. 2013 Feb;17(2):225-32. doi: 10.1111/jcmm.12027. J Cell Mol Med. 2013. PMID: 23441631 Free PMC article. Review.
-
Trpm5 channels encode bistability of spinal motoneurons and ensure motor control of hindlimbs in mice.Nat Commun. 2021 Nov 24;12(1):6815. doi: 10.1038/s41467-021-27113-x. Nat Commun. 2021. PMID: 34819493 Free PMC article.
References
-
- Aly FA, Al-Tamimi SA, Alwarthan AA. Determination of flufenamic acid and mefenamic acid in pharmaceutical preparations and biological fluids using flow injection analysis with tris(2,2′-bipyridyl)ruthenium(II) chemiluminescence detection. Anal Chim Acta. 2000;416:87–96.
-
- Anumonwo JM, Tallini YN, Vetter FJ, Jalife J. Action potential characteristics and arrhythmogenic properties of the cardiac conduction system of the murine heart. Circ Res. 2001;89:329–335. - PubMed
-
- Becq F. On the discovery and development of CFTR chloride channel activators. Curr Pharm Des. 2006;12:471–484. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

