The impact of alcohol on BCG-induced immunity against Mycobacterium tuberculosis
- PMID: 22014229
- PMCID: PMC3266991
- DOI: 10.1111/j.1530-0277.2011.01624.x
The impact of alcohol on BCG-induced immunity against Mycobacterium tuberculosis
Abstract
Background: Alcoholics are at heightened risk for developing active tuberculosis. This study evaluates chronic alcohol consumption in a murine model of vaccination with Mycobacterium bovis Bacille Calmette-Guèrin (BCG) and subsequent pulmonary infection with virulent Mycobacterium tuberculosis.
Methods: BALB/c mice were administered the Lieber-DeCarli liquid ethanol diet or pair-fed the liquid control diet for 3 weeks either before or after subcutaneous vaccination with M. bovis BCG. At least 3 weeks after BCG vaccination, groups of mice on the aforesaid diets were challenged with intratracheal infection with M. tuberculosis H37Rv. Lung mycobacterial burden, and lung and lung-associated lymph node CD4(+) lymphocyte production of tuberculosis-specific interferon (IFN)-γ were assayed. Popliteal lymph node lymphocytes from both dietary regimens undergoing BCG vaccination (in the absence of M. tuberculosis infection) were also evaluated for purified protein derivative-induced IFN-γ production by ELISpot assay.
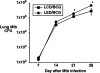
Results: Mice begun on alcohol prior to vaccination with M. bovis BCG demonstrated impaired control of pulmonary challenge with virulent M. tuberculosis, as well as impaired lung CD4(+) and popliteal lymph node T-cell IFN-γ responses. If BCG vaccination was delivered prior to initiation of alcohol feeding, the mice remained protected against a subsequent challenge with M. tuberculosis, and BCG-induced immunity was not impaired in either the lung or the popliteal lymph nodes.
Conclusions: Alcohol consumption blunts the development of the adaptive immune response to M. bovis BCG vaccination, which impairs the control of a secondary challenge with M. tuberculosis, but only if the alcohol exposure is begun prior to BCG vaccination. These results provide insight into mechanisms by which alcohol consumption impairs antimycobacterial immunity, including in response to vaccination and subsequent pathogenic challenge.
Copyright © 2011 by the Research Society on Alcoholism.
Figures








References
-
- Anderson G, Jenkinson E, Moore N, Owen J. MHC class II-positive epithelium and mesenchyme cells are both required for T-cell development in the thymus. Nature. 1993;362:70–73. - PubMed
-
- Antas PRZ, Castello-Branco LRR. New vaccines against tuberculosis: lessons learned from BCG immunization in Brazil. Trans Royal Soc Trop Med Hygiene. 2008;102:628–630. - PubMed
-
- ATS/CDC Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med. 2000;161:S221–S247. - PubMed
-
- Barber A, Coyle S, Marano M, Fischer E, Calvano S, Fong Y, Moldawer L, Lowry S. Glucocorticoid therapy alters hormonal and cytokine responses to endotoxin in man. J Immunol. 1993;150:1999–2006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

