Pharmacological characterization of rat amylin receptors: implications for the identification of amylin receptor subtypes
- PMID: 22014233
- PMCID: PMC3415645
- DOI: 10.1111/j.1476-5381.2011.01717.x
Pharmacological characterization of rat amylin receptors: implications for the identification of amylin receptor subtypes
Abstract
Background and purpose: Amylin (Amy) is an important glucoregulatory peptide and AMY receptors are clinical targets for diabetes and obesity. Human (h) AMY receptor subtypes are complexes of the calcitonin (CT) receptor with receptor activity-modifying proteins (RAMPs); their rodent counterparts have not been characterized. To allow identification of the most clinically relevant receptor subtype, the elucidation of rat (r) AMY receptor pharmacology is necessary.
Experimental approach: Receptors were transiently transfected into COS-7 cells and cAMP responses measured in response to different agonists, with or without antagonists. Competition binding experiments were performed to determine rAmy affinity.
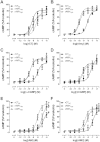
Key results: rCT was the most potent agonist of rCT((a)) receptors, whereas rAmy was most potent at rAMY(1(a)) and rAMY(3(a)) receptors. rAmy bound to these receptors with high affinity. Rat α-calcitonin gene-related peptide (CGRP) was equipotent to rAmy at both AMY receptors. Rat adrenomedullin (AM) and rAM2/intermedin activated all three receptors but were most effective at rAMY(3(a)) . AC187, AC413 and sCT(8-32) were potent antagonists at all three receptors. rαCGRP(8-37) displayed selectivity for rAMY receptors over rCT((a)) receptors. rAMY(8-37) was a weak antagonist but was more effective at rAMY(1(a)) than rAMY(3(a)) .
Conclusions and implications: AMY receptors were generated by co-expression of rCT((a)) with rRAMP1 or 3, forming rAMY(1(a)) and rAMY(3(a)) receptors, respectively. CGRP was more potent at rAMY than at hAMY receptors. No antagonist tested was able to differentiate the rAMY receptor subtypes. The data emphasize the need for and provide a useful resource for developing new CT or AMY receptor ligands as pharmacological tools or potential clinical candidates.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures



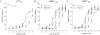

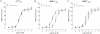
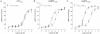

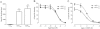




References
-
- Aiyar N, Baker E, Martin J, Patel A, Stadel JM, Willette RN, et al. Differential calcitonin gene-related peptide (CGRP) and amylin binding sites in nucleus accumbens and lung: potential models for studying CGRP/amylin receptor subtypes. J Neurochem. 1995;65:1131–1138. - PubMed
-
- Bailey RJ, Hay DL. Pharmacology of the human CGRP1 receptor in Cos 7 cells. Peptides. 2006;27:1367–1375. - PubMed
-
- Becskei C, Riediger T, Zund D, Wookey P, Lutz TA. Immunohistochemical mapping of calcitonin receptors in the adult rat brain. Brain Res. 2004;1030:221–233. - PubMed
-
- Chang CL, Roh J, Hsu SY. Intermedin, a novel calcitonin family peptide that exists in teleosts as well as in mammals: a comparison with other calcitonin/intermedin family peptides in vertebrates. Peptides. 2004;25:1633–1642. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

