The hypothalamic neuropeptide oxytocin is required for formation of the neurovascular interface of the pituitary
- PMID: 22014522
- PMCID: PMC4387193
- DOI: 10.1016/j.devcel.2011.09.004
The hypothalamic neuropeptide oxytocin is required for formation of the neurovascular interface of the pituitary
Abstract
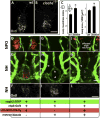
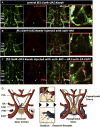
The hypothalamo-neurohypophyseal system (HNS) is the neurovascular structure through which the hypothalamic neuropeptides oxytocin and arginine-vasopressin exit the brain into the bloodstream, where they go on to affect peripheral physiology. Here, we investigate the molecular cues that regulate the neurovascular contact between hypothalamic axons and neurohypophyseal capillaries of the zebrafish. We developed a transgenic system in which both hypothalamic axons and neurohypophyseal vasculature can be analyzed in vivo. We identified the cellular organization of the zebrafish HNS as well as the dynamic processes that contribute to formation of the HNS neurovascular interface. We show that formation of this interface is regulated during development by local release of oxytocin, which affects endothelial morphogenesis. This cell communication process is essential for the establishment of a tight axovasal interface between the neurons and blood vessels of the HNS. We present a unique example of axons affecting endothelial morphogenesis through secretion of a neuropeptide.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
We declare that we have no conflicting interests.
Figures
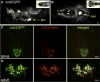
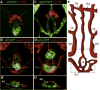
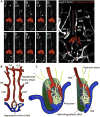
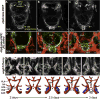

Comment in
-
The hormone of love attracts a partner for life.Dev Cell. 2011 Oct 18;21(4):602-4. doi: 10.1016/j.devcel.2011.09.018. Dev Cell. 2011. PMID: 22014517
Similar articles
-
The metabolic regulator PGC-1α directly controls the expression of the hypothalamic neuropeptide oxytocin.J Neurosci. 2011 Oct 19;31(42):14835-40. doi: 10.1523/JNEUROSCI.1798-11.2011. J Neurosci. 2011. PMID: 22016516 Free PMC article.
-
Neuropeptide oxytocin facilitates its own brain-to-periphery uptake.Cell Rep. 2025 Apr 22;44(4):115491. doi: 10.1016/j.celrep.2025.115491. Epub 2025 Apr 3. Cell Rep. 2025. PMID: 40184254
-
Neuropeptide cells and fibers in the hypothalamus and pituitary of the fetal sheep: comparison of oxytocin and arginine vasopressin.Neuroendocrinology. 1989 Dec;50(6):633-43. doi: 10.1159/000125292. Neuroendocrinology. 1989. PMID: 2515463
-
Specific expression of optically active reporter gene in arginine vasopressin-secreting neurosecretory cells in the hypothalamic-neurohypophyseal system.J Neuroendocrinol. 2008 Jun;20(6):660-4. doi: 10.1111/j.1365-2826.2008.01706.x. J Neuroendocrinol. 2008. PMID: 18601686 Review.
-
Central nervous system effects of the neurohypophyseal hormones and related peptides.Front Neuroendocrinol. 1993 Oct;14(4):251-302. doi: 10.1006/frne.1993.1009. Front Neuroendocrinol. 1993. PMID: 8258377 Review.
Cited by
-
A ligand-receptor interactome atlas of the zebrafish.iScience. 2023 Jul 12;26(8):107309. doi: 10.1016/j.isci.2023.107309. eCollection 2023 Aug 18. iScience. 2023. PMID: 37539027 Free PMC article.
-
Direct and indirect roles of Fgf3 and Fgf10 in innervation and vascularisation of the vertebrate hypothalamic neurohypophysis.Development. 2013 Mar;140(5):1111-22. doi: 10.1242/dev.080226. Development. 2013. PMID: 23404108 Free PMC article.
-
Evolution of oxytocin pathways in the brain of vertebrates.Front Behav Neurosci. 2014 Feb 14;8:31. doi: 10.3389/fnbeh.2014.00031. eCollection 2014. Front Behav Neurosci. 2014. PMID: 24592219 Free PMC article. Review.
-
Novel hypophysiotropic AgRP2 neurons and pineal cells revealed by BAC transgenesis in zebrafish.Sci Rep. 2017 Mar 20;7:44777. doi: 10.1038/srep44777. Sci Rep. 2017. PMID: 28317906 Free PMC article.
-
Role of developmental factors in hypothalamic function.Front Neuroanat. 2015 Apr 21;9:47. doi: 10.3389/fnana.2015.00047. eCollection 2015. Front Neuroanat. 2015. PMID: 25954163 Free PMC article. Review.
References
-
- Amer S, Brown JA. Glomerular actions of arginine vasotocin in the in situ perfused trout kidney. Am J Physiol. 1995;269:R775–R780. - PubMed
-
- Bargmann W. J Mol Med. 1949;27:617–622.
-
- Billenstien DC, Leveque TF. The reorganization of the neurohypophyseal stalk following hypophysectomy in the rat. Endocrinology. 1955;56:704–717. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

