Dual roles for Rac2 in neutrophil motility and active retention in zebrafish hematopoietic tissue
- PMID: 22014524
- PMCID: PMC3199325
- DOI: 10.1016/j.devcel.2011.07.013
Dual roles for Rac2 in neutrophil motility and active retention in zebrafish hematopoietic tissue
Abstract
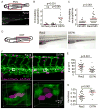
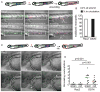
Neutrophil homeostasis is essential for host defense. Here we identify dual roles for Rac2 during neutrophil homeostasis using a zebrafish model of primary immune deficiency induced by the human inhibitory Rac2D57N mutation in neutrophils. Noninvasive live imaging of Rac2 morphants or Rac2D57N zebrafish larvae demonstrates an essential role for Rac2 in regulating 3D motility and the polarization of F-actin dynamics and PI(3)K signaling in vivo. Tracking of photolabeled Rac2-deficient neutrophils from hematopoietic tissue also shows increased mobilization into the circulation, indicating that neutrophil mobilization does not require traditionally defined cell motility. Moreover, excessive neutrophil retention in hematopoietic tissue resulting from a constitutively active CXCR4 mutation in zebrafish warts, hypogammaglobulinemia, infections, and myelokathexis (WHIM) syndrome is partially rescued by the inhibitory Rac2 mutation. These findings reveal that Rac2 signaling is necessary for both neutrophil 3D motility and CXCR4-mediated neutrophil retention in hematopoietic tissue, thereby limiting neutrophil mobilization, a critical first step in the innate immune response.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
-
- Accetta D, Syverson G, Bonacci B, Reddy S, Bengtson C, Surfus J, Harbeck R, Huttenlocher A, Grossman W, Routes J, Verbsky J. Human phagocyte defect caused by a Rac2 mutation detected by means of neonatal screening for T-cell lymphopenia. J Allergy Clin Immunol 2010 - PubMed
-
- Alberts AS, Qin H, Carr HS, Frost JA. PAK1 negatively regulates the activity of the Rho exchange factor NET1. J Biol Chem. 2005;280:12152–12161. - PubMed
-
- Alkhatib G, Berger EA. HIV coreceptors: from discovery and designation to new paradigms and promise. Eur J Med Res. 2007;12:375–384. - PubMed
-
- Berthier E, Surfus J, Verbsky J, Huttenlocher A, Beebe D. An arrayed high-content chemotaxis assay for patient diagnosis. Integr Biol (Camb) 2010;2:630–638. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

