A muscle-specific p38 MAPK/Mef2/MnSOD pathway regulates stress, motor function, and life span in Drosophila
- PMID: 22014527
- PMCID: PMC3199449
- DOI: 10.1016/j.devcel.2011.09.002
A muscle-specific p38 MAPK/Mef2/MnSOD pathway regulates stress, motor function, and life span in Drosophila
Abstract
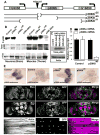
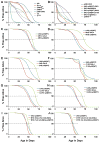
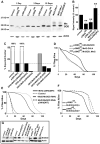
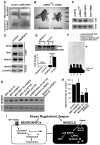
Molecular mechanisms that concordantly regulate stress, life span, and aging remain incompletely understood. Here, we demonstrate that in Drosophila, a p38 MAP kinase (p38K)/Mef2/MnSOD pathway is a coregulator of stress and life span. Hence, overexpression of p38K extends life span in a MnSOD-dependent manner, whereas inhibition of p38K causes early lethality and precipitates age-related motor dysfunction and stress sensitivity, that is rescued through muscle-restricted (but not neuronal) add-back of p38K. Additionally, mutations in p38K are associated with increased protein carbonylation and Nrf2-dependent transcription, while adversely affecting metabolic response to hypoxia. Mechanistically, p38K modulates expression of the mitochondrial MnSOD enzyme through the transcription factor Mef2, and predictably, perturbations in MnSOD modify p38K-dependent phenotypes. Thus, our results uncover a muscle-restricted p38K-Mef2-MnSOD signaling module that influences life span and stress, distinct from the insulin/JNK/FOXO pathway. We propose that potentiating p38K might be instrumental in restoring the mitochondrial detoxification machinery and combating stress-induced aging.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Evolutionarily conserved transcription factors drive the oxidative stress response in Drosophila.J Exp Biol. 2020 Jul 20;223(Pt 14):jeb221622. doi: 10.1242/jeb.221622. J Exp Biol. 2020. PMID: 32532866 Free PMC article.
-
p38 MAP kinase regulates circadian rhythms in Drosophila.J Biol Rhythms. 2014 Dec;29(6):411-26. doi: 10.1177/0748730414555183. Epub 2014 Nov 17. J Biol Rhythms. 2014. PMID: 25403440
-
JNK/FOXO-mediated neuronal expression of fly homologue of peroxiredoxin II reduces oxidative stress and extends life span.J Biol Chem. 2009 Oct 23;284(43):29454-61. doi: 10.1074/jbc.M109.028027. Epub 2009 Aug 31. J Biol Chem. 2009. PMID: 19720829 Free PMC article.
-
JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling.Cell. 2005 Apr 8;121(1):115-25. doi: 10.1016/j.cell.2005.02.030. Cell. 2005. PMID: 15820683
-
Hydrogen peroxide mediates hyperglycemia-induced invasive activity via ERK and p38 MAPK in human pancreatic cancer.Oncotarget. 2015 Oct 13;6(31):31119-33. doi: 10.18632/oncotarget.5045. Oncotarget. 2015. PMID: 26439801 Free PMC article.
Cited by
-
MKK3 modulates JNK-dependent cell migration and invasion.Cell Death Dis. 2019 Feb 15;10(3):149. doi: 10.1038/s41419-019-1350-6. Cell Death Dis. 2019. PMID: 30770795 Free PMC article.
-
Iron Sulfur and Molybdenum Cofactor Enzymes Regulate the Drosophila Life Cycle by Controlling Cell Metabolism.Front Physiol. 2018 Feb 14;9:50. doi: 10.3389/fphys.2018.00050. eCollection 2018. Front Physiol. 2018. PMID: 29491838 Free PMC article.
-
Functional conservation in genes and pathways linking ageing and immunity.Immun Ageing. 2021 May 14;18(1):23. doi: 10.1186/s12979-021-00232-1. Immun Ageing. 2021. PMID: 33990202 Free PMC article. Review.
-
Chromatin insulator bodies are nuclear structures that form in response to osmotic stress and cell death.J Cell Biol. 2013 Jul 22;202(2):261-76. doi: 10.1083/jcb.201304181. J Cell Biol. 2013. PMID: 23878275 Free PMC article.
-
A survey of the protective effects of some commercially available antioxidant supplements in genetically and chemically induced models of oxidative stress in Drosophila melanogaster.Exp Gerontol. 2012 Sep;47(9):712-22. doi: 10.1016/j.exger.2012.06.016. Epub 2012 Jul 10. Exp Gerontol. 2012. PMID: 22790021 Free PMC article.
References
-
- Adachi-Yamada T, Nakamura M, Irie K, Tomoyasu Y, Sano Y, Mori E, Goto S, Ueno N, Nishida Y, Matsumoto K. p38 mitogen-activated protein kinase can be involved in transforming growth factor beta superfamily signal transduction in Drosophila wing morphogenesis. Mol Cell Biol. 1999;19:2322–2329. - PMC - PubMed
-
- Andreadi CK, Howells LM, Atherfold PA, Manson MM. Involvement of Nrf2, p38, B-Raf, and nuclear factor-kappaB, but not phosphatidylinositol 3-kinase, in induction of hemeoxygenase-1 by dietary polyphenols. Mol Pharmacol. 2006;69:1033–1040. - PubMed
-
- Ashwell JD. The many paths to p38 mitogen-activated protein kinase activation in the immune system. Nat Rev Immunol. 2006;6:532–540. - PubMed
-
- Barbieri M, Bonafe M, Franceschi C, Paolisso G. Insulin/IGF-I-signaling pathway: an evolutionarily conserved mechanism of longevity from yeast to humans. Am J Physiol Endocrinol Metab. 2003;285:E1064–1071. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

