Intensive intervention for alcohol-dependent smokers in early recovery: a randomized trial
- PMID: 22014532
- PMCID: PMC3288470
- DOI: 10.1016/j.drugalcdep.2011.09.026
Intensive intervention for alcohol-dependent smokers in early recovery: a randomized trial
Abstract
Introduction: The purpose of this study was to investigate the efficacy of an intensive tobacco cessation intervention for alcohol-dependent smokers in early recovery.
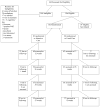
Methods: A total of 162 alcohol-dependent smokers were randomized to either intensive intervention for smoking cessation or usual care. The intensive intervention consisted of 16 sessions of individual cognitive behavior therapy (CBT) and combination nicotine replacement therapy that lasted 26 weeks. Usual care involved referral to a free-standing smoking cessation program that provided smoking cessation counseling of varying duration and guideline-concordant medications. The primary cessation outcome was verified 7-day point prevalence abstinence (PPA) at 12, 26, 38, and 52 weeks.
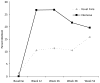
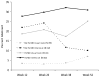
Results: At 12 and 26 weeks, the verified 7-day point-prevalence quit rate was significantly higher for the intensive intervention group than for the usual care group (both p=0.03). However, the quit rates for the two treatment groups were not significantly different at 38 or 52 weeks. Verified 30-day alcohol abstinence rates were not significantly different for the two treatment groups at any of the follow-up assessments.
Conclusions: The intensive smoking cessation intervention yielded a higher short-term smoking quit rate without jeopardizing sobriety. A chronic care model might facilitate maintenance of smoking cessation during the first year of alcohol treatment and perhaps for longer periods of time. It is hoped that studies such as this will inform the development of more effective interventions for concurrent alcohol and tobacco use disorders.
Published by Elsevier Ireland Ltd.
Conflict of interest statement
There are no disclosures for any authors.
Figures
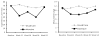

References
-
- Appleby L, Dyson V, Altman E, Luchins DJ. Assessing substance use in multiproblem patients: reliability and validity of the Addiction Severity Index in a mental hospital population. J Nerv Ment Dis. 1997;185:159–165. - PubMed
-
- Baca CT, Yahne CE. Smoking cessation during substance abuse treatment: what you need to know. J Subst Abuse Treat. 2009;36:205–219. - PubMed
-
- Beck AT, Steer RA. Internal consistencies of the original and revised Beck Depression Inventory. J Clin Psychol. 1984;40:1365–1367. - PubMed
-
- Beck AT, Ward CH, Mendelsohn M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. - PubMed
-
- Blalock JA, Robinson JD, Wetter DW, Schreindorfer LS, Cinciripini PM. Nicotine withdrawal in smokers with current depressive disorders undergoing intensive smoking cessation treatment. Psychol Addict Behav. 2008;22:122–128. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

