Renal cyst formation in Fh1-deficient mice is independent of the Hif/Phd pathway: roles for fumarate in KEAP1 succination and Nrf2 signaling
- PMID: 22014577
- PMCID: PMC3202623
- DOI: 10.1016/j.ccr.2011.09.006
Renal cyst formation in Fh1-deficient mice is independent of the Hif/Phd pathway: roles for fumarate in KEAP1 succination and Nrf2 signaling
Abstract
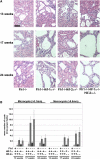
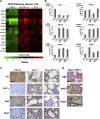
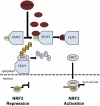
The Krebs cycle enzyme fumarate hydratase (FH) is a human tumor suppressor whose inactivation is associated with the development of leiomyomata, renal cysts, and tumors. It has been proposed that activation of hypoxia inducible factor (HIF) by fumarate-mediated inhibition of HIF prolyl hydroxylases drives oncogenesis. Using a mouse model, we provide genetic evidence that Fh1-associated cyst formation is Hif independent, as is striking upregulation of antioxidant signaling pathways revealed by gene expression profiling. Mechanistic analysis revealed that fumarate modifies cysteine residues within the Kelch-like ECH-associated protein 1 (KEAP1), abrogating its ability to repress the Nuclear factor (erythroid-derived 2)-like 2 (Nrf2)-mediated antioxidant response pathway, suggesting a role for Nrf2 dysregulation in FH-associated cysts and tumors.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures




Comment in
-
Succination of Keap1 and activation of Nrf2-dependent antioxidant pathways in FH-deficient papillary renal cell carcinoma type 2.Cancer Cell. 2011 Oct 18;20(4):418-20. doi: 10.1016/j.ccr.2011.10.005. Cancer Cell. 2011. PMID: 22014567 Free PMC article.
-
Kidney tumours. 'NRF said.Nat Rev Cancer. 2011 Nov 10;11(12):833. doi: 10.1038/nrc3175. Nat Rev Cancer. 2011. PMID: 22071979 No abstract available.
References
-
- Alderson N.L., Wang Y., Blatnik M., Frizzell N., Walla M.D., Lyons T.J., Alt N., Carson J.A., Nagai R., Thorpe S.R., Baynes J.W. S-(2-Succinyl)cysteine: a novel chemical modification of tissue proteins by a Krebs cycle intermediate. Arch. Biochem. Biophys. 2006;450:1–8. - PubMed
-
- Aragonés J., Schneider M., Van Geyte K., Fraisl P., Dresselaers T., Mazzone M., Dirkx R., Zacchigna S., Lemieux H., Jeoung N.H. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat. Genet. 2008;40:170–180. - PubMed
-
- Ashrafian H., O'Flaherty L., Adam J., Steeples V., Chung Y.L., East P., Vanharanta S., Lehtonen H., Nye E., Hatipoglu E. Expression profiling in progressive stages of fumarate-hydratase deficiency: the contribution of metabolic changes to tumorigenesis. Cancer Res. 2010;70:9153–9165. - PubMed
-
- Bardella C., El-Bahrawy M., Frizzell N., Adam J., Ternette N., Hatipoglu E., Howarth K., O'Flaherty L., Roberts I., Turner G. Aberrant succination of proteins in fumarate hydratase-deficient mice and HLRCC patients is a robust biomarker of mutation status. J. Pathol. 2011;225:4–11. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

