Cofactor regulation of C5a chemotactic activity in physiological fluids. Requirement for the vitamin D binding protein, thrombospondin-1 and its receptors
- PMID: 22014686
- PMCID: PMC3224156
- DOI: 10.1016/j.molimm.2011.09.024
Cofactor regulation of C5a chemotactic activity in physiological fluids. Requirement for the vitamin D binding protein, thrombospondin-1 and its receptors
Abstract
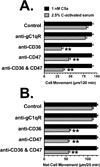
Factors in physiological fluids that regulate the chemotactic activity of complement activation peptides C5a and C5a des Arg are not well understood. The vitamin D binding protein (DBP) has been shown to significantly enhance chemotaxis to C5a/C5a des Arg. More recently, platelet-derived thrombospondin-1 (TSP-1) has been shown to facilitate the augmentation of C5a-induced chemotaxis by DBP. The objective of this study was to better characterize these chemotactic cofactors and investigate the role that cell surface TSP-1 receptors CD36 and CD47 may play in this process. The chemotactic activity in C-activated normal serum, citrated plasma, DBP-depleted serum or C5 depleted serum was determined for both normal human neutrophils and U937 cell line transfected with the C5a receptor (U937-C5aR). In addition, levels of C5a des Arg, DBP and TSP-1 in these fluids were measured by RIA or ELISA. Results show that there is a clear hierarchy with C5a being the essential primary signal (DBP or TSP-1 will not function in the absence of C5a), DBP the necessary cofactor and TSP-1 a dependent tertiary factor, since it cannot function to enhance chemotaxis to C5a without DBP. Measurement of the C5a-induced intracellular calcium flux confirmed the same hierarchy observed with chemotaxis. Moreover, analysis of bronchoalveolar lavage fluid (BALF) from patients with the adult respiratory distress syndrome (ARDS) demonstrated that C5a-dependent chemotactic activity is significantly decreased after anti-DBP treatment. Finally, results show that TSP-1 utilizes cell surface receptors CD36 and CD47 to augment chemotaxis, but DBP does not bind to TSP-1, CD36 or CD47. The results clearly demonstrate that C5a/C5a des Arg needs both DBP and TSP-1 for maximal chemotactic activity and suggest that the regulation of C5a chemotactic activity in physiological fluids is more complex than previously thought.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures






References
-
- Alessio M, De Monte L, Scirea A, Gruarin P, Tandon NN, Sitia R. Synthesis, processing, and intracellular transport of CD36 during monocytic differentiation. J Biol Chem. 1996;271:1770–1775. - PubMed
-
- Binder R, Kress A, Kan G, Herrmann K, Kirschfink M. Neutrophil priming by cytokines and vitamin D binding protein (Gc-globulin): impact on C5a-mediated chemotaxis, degranulation and respiratory burst. Mol Immunol. 1999;36:885–892. - PubMed
-
- Brown EJ, Frazier WA. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11:130–135. - PubMed
-
- Daviet L, Malvoisin E, Wild TF, McGregor JL. Thrombospondin induces dimerization of membrane-bound, but not soluble CD36. Thrombosis and haemostasis. 1997;78:897–901. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

