Pharmacophore-based discovery of FXR-agonists. Part II: identification of bioactive triterpenes from Ganoderma lucidum
- PMID: 22014750
- PMCID: PMC3254236
- DOI: 10.1016/j.bmc.2011.09.039
Pharmacophore-based discovery of FXR-agonists. Part II: identification of bioactive triterpenes from Ganoderma lucidum
Abstract
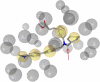
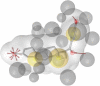
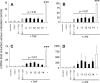
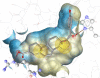
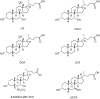
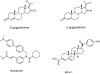
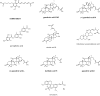
The farnesoid X receptor (FXR) belonging to the metabolic subfamily of nuclear receptors is a ligand-induced transcriptional activator. Its central function is the physiological maintenance of bile acid homeostasis including the regulation of glucose and lipid metabolism. Accessible structural information about its ligand-binding domain renders FXR an attractive target for in silico approaches. Integrated to natural product research these computational tools assist to find novel bioactive compounds showing beneficial effects in prevention and treatment of, for example, the metabolic syndrome, dyslipidemia, atherosclerosis, and type 2 diabetes. Virtual screening experiments of our in-house Chinese Herbal Medicine database with structure-based pharmacophore models, previously generated and validated, revealed mainly lanostane-type triterpenes of the TCM fungus Ganoderma lucidum Karst. as putative FXR ligands. To verify the prediction of the in silico approach, two Ganoderma fruit body extracts and compounds isolated thereof were pharmacologically investigated. Pronounced FXR-inducing effects were observed for the extracts at a concentration of 100 μg/mL. Intriguingly, five lanostanes out of 25 secondary metabolites from G. lucidum, that is, ergosterol peroxide (2), lucidumol A (11), ganoderic acid TR (12), ganodermanontriol (13), and ganoderiol F (14), dose-dependently induced FXR in the low micromolar range in a reporter gene assay. To rationalize the binding interactions, additional pharmacophore profiling and molecular docking studies were performed, which allowed establishing a first structure-activity relationship of the investigated triterpenes.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
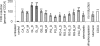
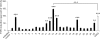

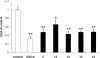
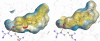



References
-
- Forman B.M., Goode E., Chen J., Oro A.E., Bradley D.J., Perlmann T., Noonan D.J., Burka L.T., McMorris T., Lamph W.W., Evans R.M., Weinberger C. Cell. 1995;81:687. - PubMed
-
- Fiorucci S., Rizzo G., Donini A., Distrutti E., Santucci L. Trends Mol. Med. 2007;13:298. - PubMed
-
- Pellicciari R., Costantino G., Fiorucci S. J. Med. Chem. 2005;48:5383. - PubMed
-
- Shen H., Zhang Y., Ding H., Wang X., Chen L., Jiang H., Shen X. Cell. Physiol. Biochem. 2008;22:1. - PubMed
-
- Makishima M., Okamoto A.Y., Repa J.J., Tu H., Learned R.M., Luk A., Hull M.V., Lustig K.D., Mangelsdorf D.J., Shan B. Science. 1999;284:1362. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

