Toremifene decreases vertebral fractures in men younger than 80 years receiving androgen deprivation therapy for prostate cancer
- PMID: 22014807
- PMCID: PMC3671875
- DOI: 10.1016/j.juro.2011.07.090
Toremifene decreases vertebral fractures in men younger than 80 years receiving androgen deprivation therapy for prostate cancer
Abstract
Purpose: Androgen deprivation therapy is associated with an increased fracture risk. In a recent phase III trial toremifene significantly decreased vertebral fractures in men on androgen deprivation therapy. Similar to other selective estrogen receptor modulators, toremifene was associated with an increase in venous thromboembolic events with the greatest risk in men 80 years old or older. In this post hoc analysis we evaluated the efficacy and safety of toremifene in men younger than 80 years.
Materials and methods: This analysis included 847 men younger than 80 years, of whom 430 received toremifene 80 mg by mouth daily and 417 received placebo for up to 24 months. The primary end point was new vertebral fractures. Secondary end points included fragility fractures, bone mineral density and safety.
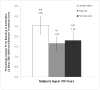
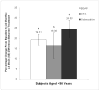
Results: Compared with placebo, toremifene decreased the relative risk of new vertebral fractures by 79.5% (95% CI 29.8-94.0, p <0.005). The new vertebral fracture incidence was 1.0% for toremifene and 4.8% for placebo (absolute risk reduction 3.8%). Compared with placebo, toremifene significantly decreased the incidence of nontraumatic fracture or greater than 7% bone loss by 24 months (p <0.0001). Toremifene also significantly increased bone mineral density at all measured sites (all comparisons p <0.001). The incidence of venous thromboembolic events was similar in the toremifene and placebo groups (2.1% and 1.0%, respectively, p = 0.26). The rates of other adverse events were also similar between the groups.
Conclusions: Toremifene significantly decreased new vertebral fractures in men younger than 80 years receiving androgen deprivation therapy for prostate cancer. The risk of venous thromboembolic events was lower than in the overall study population, suggesting an improved risk-benefit profile in younger men.
Copyright © 2011 American Urological Association Education and Research, Inc. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
Similar articles
-
[Osteoporosis in men receiving androgen deprivation therapy for non-metastatic prostate cancer].Wien Med Wochenschr. 2012 Sep;162(17-18):380-5. doi: 10.1007/s10354-012-0106-z. Epub 2012 Aug 9. Wien Med Wochenschr. 2012. PMID: 22875632 Review. German.
-
Toremifene to reduce fracture risk in men receiving androgen deprivation therapy for prostate cancer.J Urol. 2010 Oct;184(4):1316-21. doi: 10.1016/j.juro.2010.06.022. Epub 2010 Aug 17. J Urol. 2010. PMID: 20723926 Free PMC article. Clinical Trial.
-
Toremifene to reduce fracture risk in men receiving androgen deprivation therapy for prostate cancer.J Urol. 2013 Jan;189(1 Suppl):S45-50. doi: 10.1016/j.juro.2012.11.016. J Urol. 2013. PMID: 23234631 Clinical Trial.
-
Factors associated with vertebral fractures in men treated with androgen deprivation therapy for prostate cancer.J Urol. 2011 Aug;186(2):482-6. doi: 10.1016/j.juro.2011.03.111. Epub 2011 Jun 15. J Urol. 2011. PMID: 21679977 Free PMC article. Clinical Trial.
-
Toremifene--a promising therapy for the prevention of prostate cancer and complications of androgen deprivation therapy.Expert Opin Investig Drugs. 2006 Mar;15(3):293-305. doi: 10.1517/13543784.15.3.293. Expert Opin Investig Drugs. 2006. PMID: 16503765 Review.
Cited by
-
The skeletal impact of cancer therapies.Br J Clin Pharmacol. 2019 Jun;85(6):1161-1168. doi: 10.1111/bcp.13866. Epub 2019 Feb 21. Br J Clin Pharmacol. 2019. PMID: 30723928 Free PMC article. Review.
-
Battle of the sex steroids in the male skeleton: and the winner is...J Clin Invest. 2016 Mar 1;126(3):829-32. doi: 10.1172/JCI85006. Epub 2016 Feb 22. J Clin Invest. 2016. PMID: 26901810 Free PMC article.
-
Androgen Receptor-CaMKK2 Axis in Prostate Cancer and Bone Microenvironment.Front Endocrinol (Lausanne). 2018 Jun 18;9:335. doi: 10.3389/fendo.2018.00335. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29967592 Free PMC article. Review.
-
Osteoporosis in men.J Osteoporos. 2012;2012:675984. doi: 10.1155/2012/675984. Epub 2012 Apr 12. J Osteoporos. 2012. PMID: 22570804 Free PMC article. No abstract available.
-
[Osteoporosis in men receiving androgen deprivation therapy for non-metastatic prostate cancer].Wien Med Wochenschr. 2012 Sep;162(17-18):380-5. doi: 10.1007/s10354-012-0106-z. Epub 2012 Aug 9. Wien Med Wochenschr. 2012. PMID: 22875632 Review. German.
References
-
- Prostate Cancer. V1.2010. Fort Washington, PA: National Comprehensive Cancer Network; 2009. Clinical Practice Guidelines in Oncology™.
-
- Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

